Biochemistry Preparation of soap Soap sodium or potassium

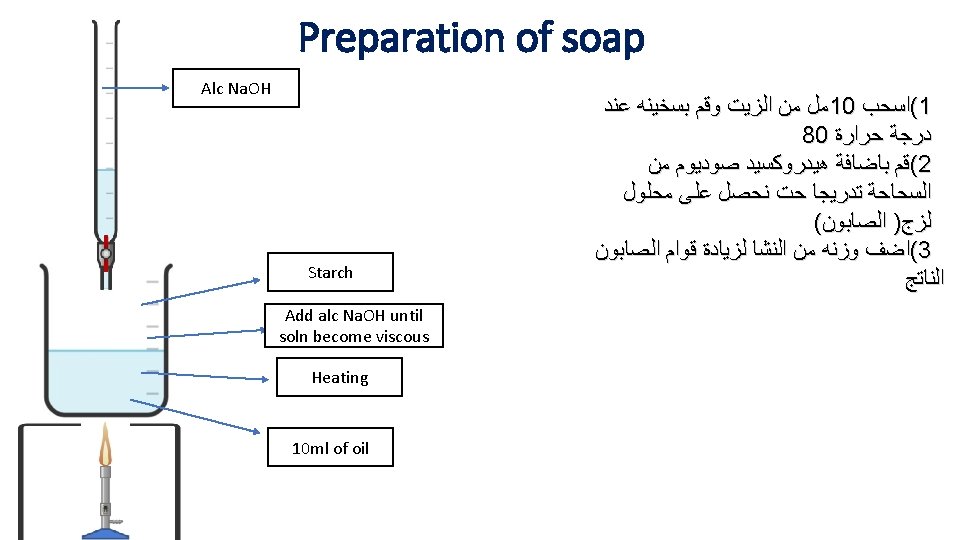
Biochemistry Preparation of soap
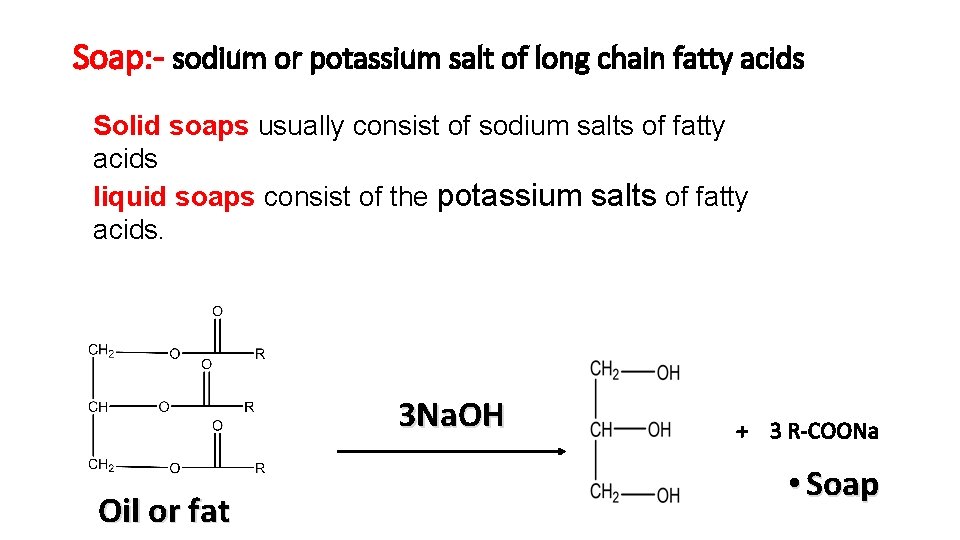
Soap: - sodium or potassium salt of long chain fatty acids Solid soaps usually consist of sodium salts of fatty acids liquid soaps consist of the potassium salts of fatty acids. 3 Na. OH Oil or fat + 3 R-COONa • Soap

Properties of soap 1) Separation of fatty acids Oily drops on the surface 2) salting out the soap will precipitate Warming . 5 ml conc HCl Na. Cl 5 ml of soap soln R-COONa + HCl 5 ml of soap soln R-COOH + HCl R-COONa Na. Cl
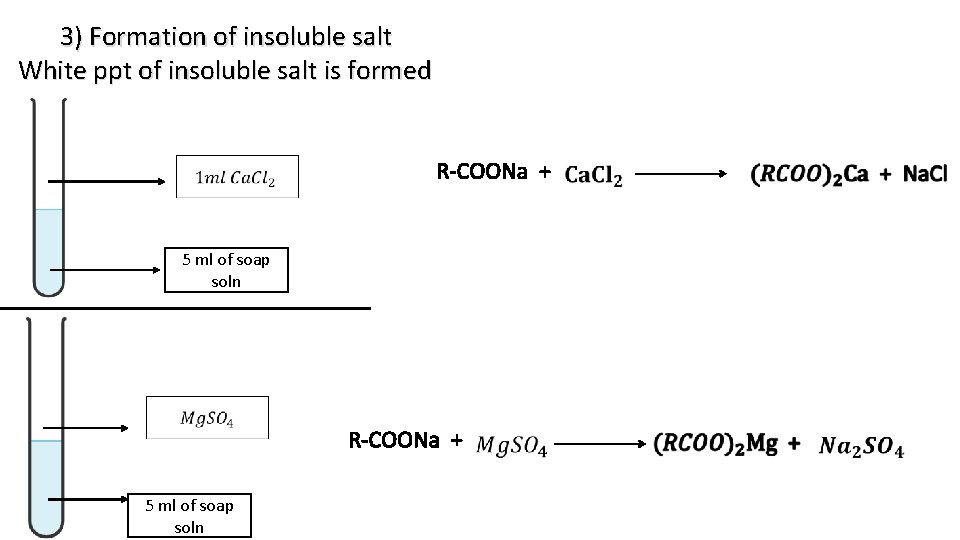
3) Formation of insoluble salt White ppt of insoluble salt is formed R-COONa + 5 ml of soap soln

Analysis of soap •
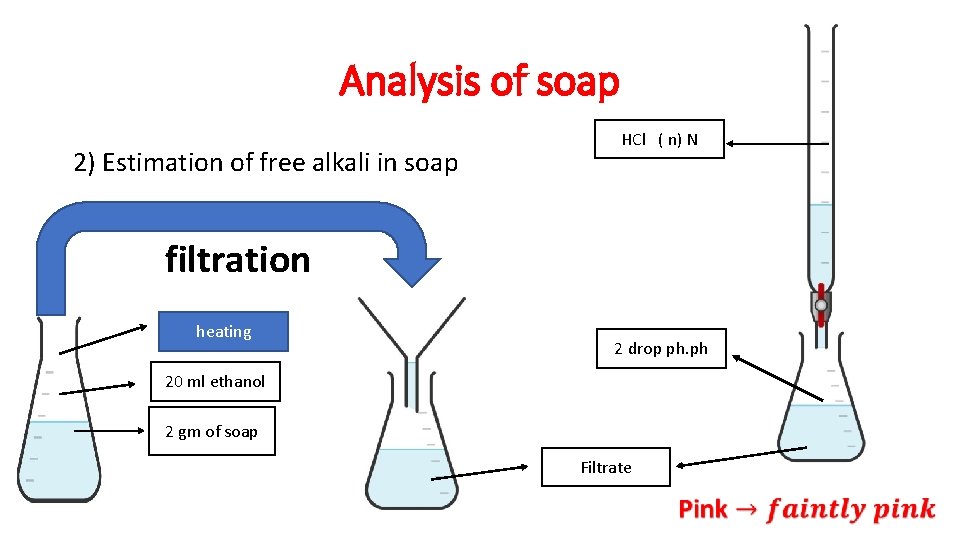
Analysis of soap 2) Estimation of free alkali in soap HCl ( n) N filtration heating 2 drop ph. ph 20 ml ethanol 2 gm of soap Filtrate
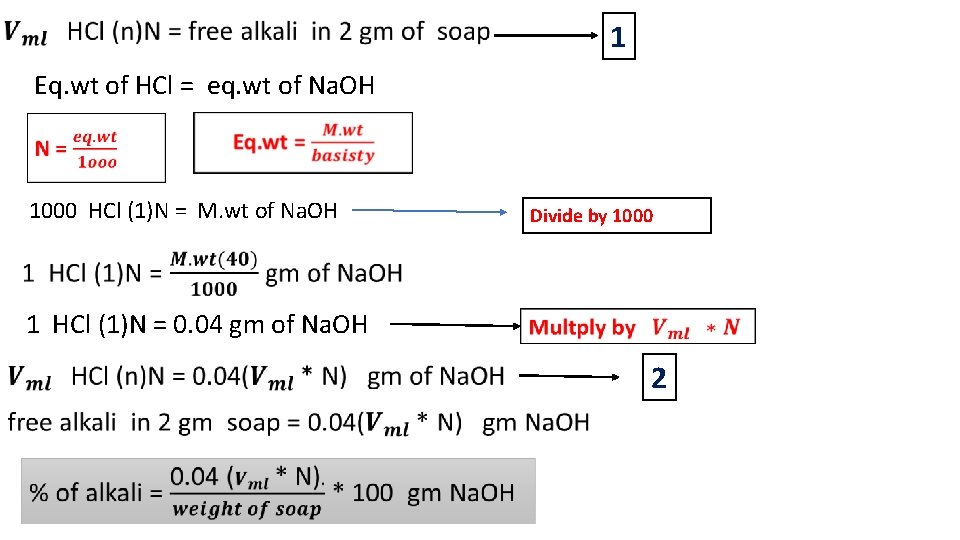
1 Eq. wt of HCl = eq. wt of Na. OH 1000 HCl (1)N = M. wt of Na. OH Divide by 1000 1 HCl (1)N = 0. 04 gm of Na. OH 2
- Slides: 8