BIOCHEMISTRY ORGANIC MOLECULES CONTAIN CARBON AND HYDROGEN CARBONS

BIOCHEMISTRY

ORGANIC MOLECULES • CONTAIN CARBON AND HYDROGEN -CARBON’S ABILITY TO BOND WITH 4 DIFFERENT ATOMS ALLOWS IT TO FORM LARGE MOLECULES

MACROMOLECULES • CARBOHYDRATES • LIPIDS THESE MOLECUL ES ARE THE BUILDING BLOCKS OF ALL LIFE!!! • NUCLEIC ACIDS • PROTEINS

CARBOHYDRATES CARBON HYDROGEN OXYGEN 1: 2: 1 MONOMER=MONOSACCHARIDE EX: GLUCOSE CARBOHYDRATES HAVE A STRUCTURE CONTAINING A CARBON BACKBONE
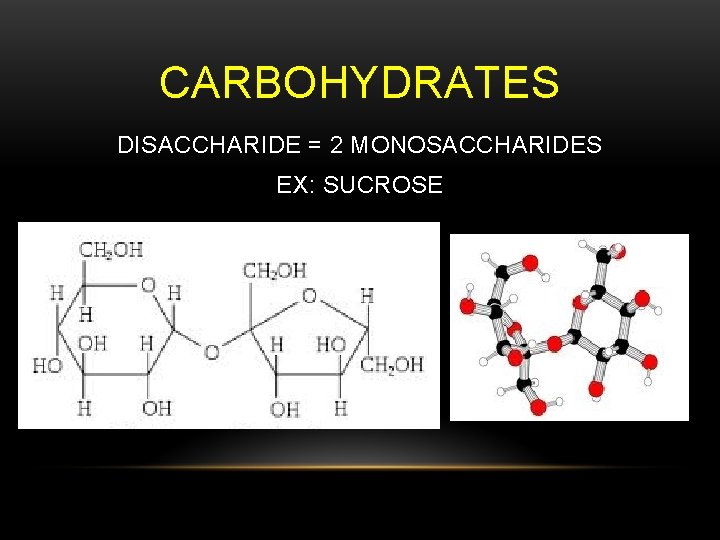
CARBOHYDRATES DISACCHARIDE = 2 MONOSACCHARIDES EX: SUCROSE
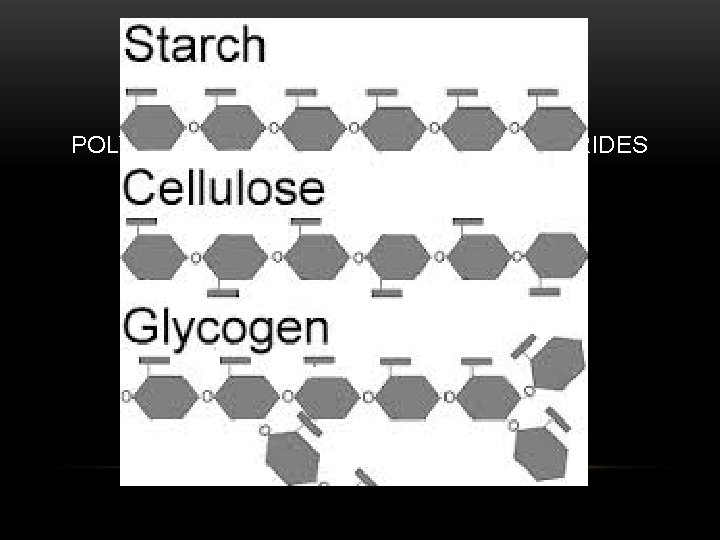
CARBOHYDRATES POLYCCHARIDE = 100 S OF MONOSACCHARIDES EX: STARCH, CELLULOSE, GLYCOGEN

CARBOHYDRATES ARE USED TO STORE ENERGY IN ORGANISMS -CELLULOSE IS THE MAIN BUILDING MATERIAL OF PLANTS, AND IS MADE WHEN SHEETS OF GLUCOSE MOLECULES ARE CONNECTED BY HYDROGEN BONDS -STARCH IS USED FOR LONG TERM ENERGY STORAGE IN PLANTS -GLYCOGEN IS AN ENERGY STORAGE MOLECULE FOUND IN ANIMALS AND FUNGI
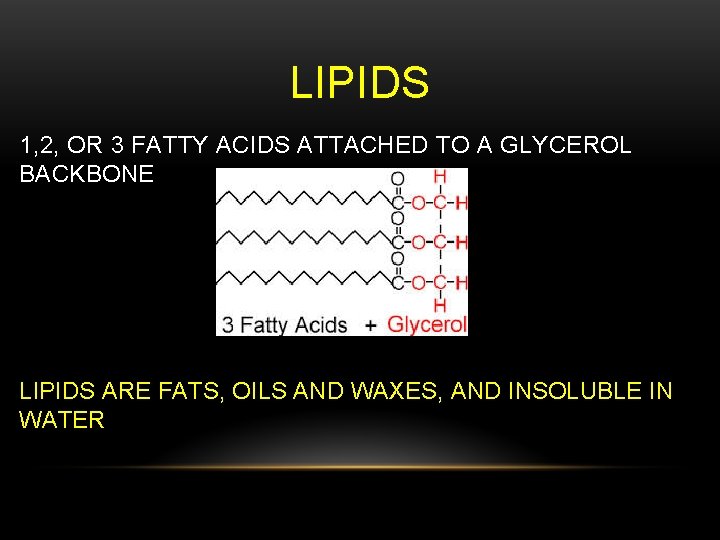
LIPIDS 1, 2, OR 3 FATTY ACIDS ATTACHED TO A GLYCEROL BACKBONE LIPIDS ARE FATS, OILS AND WAXES, AND INSOLUBLE IN WATER
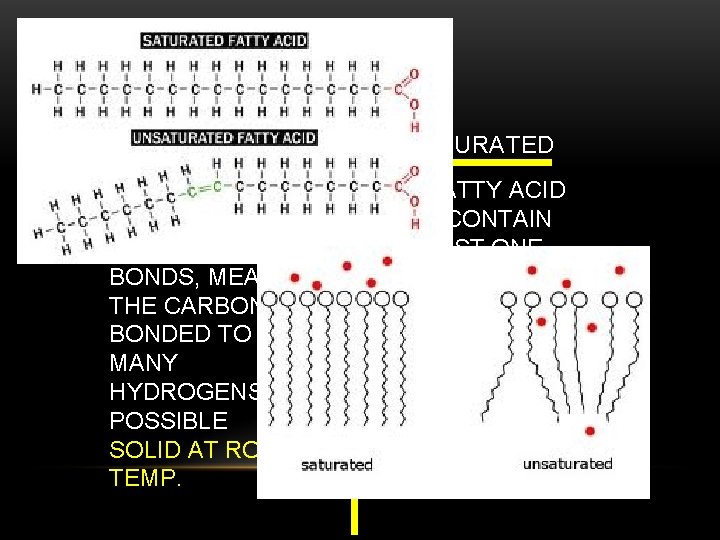
LIPIDS SATURATED VS UNSATURATED THE FATTY ACID TAILS CONTAIN ONLY SINGLE BONDS, MEANING THE CARBON IS BONDED TO AS MANY HYDROGENS AS POSSIBLE SOLID AT ROOM TEMP. THE FATTY ACID TAILS CONTAIN AT LEAST ONE CARBON TO CARBON DOUBLE BOND LIQUID AT ROOM TEMP.
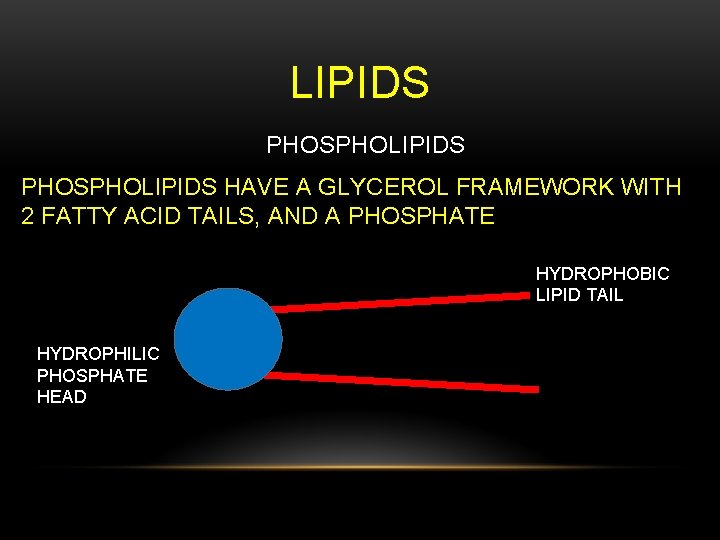
LIPIDS PHOSPHOLIPIDS HAVE A GLYCEROL FRAMEWORK WITH 2 FATTY ACID TAILS, AND A PHOSPHATE HYDROPHOBIC LIPID TAIL HYDROPHILIC PHOSPHATE HEAD
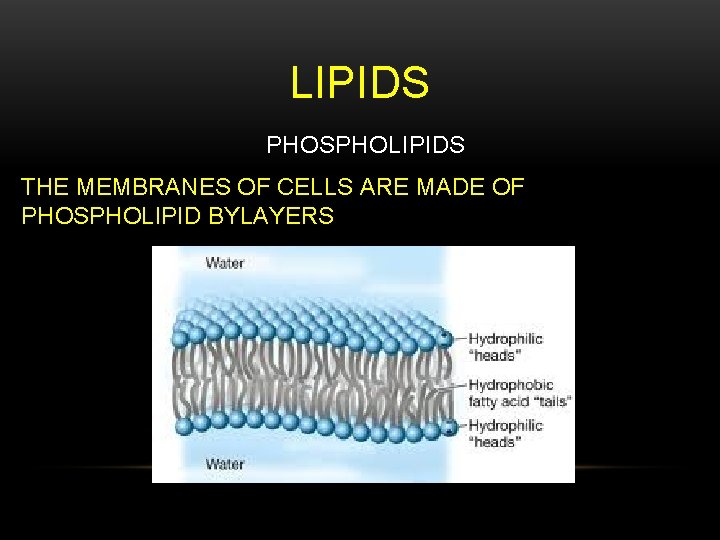
LIPIDS PHOSPHOLIPIDS THE MEMBRANES OF CELLS ARE MADE OF PHOSPHOLIPID BYLAYERS
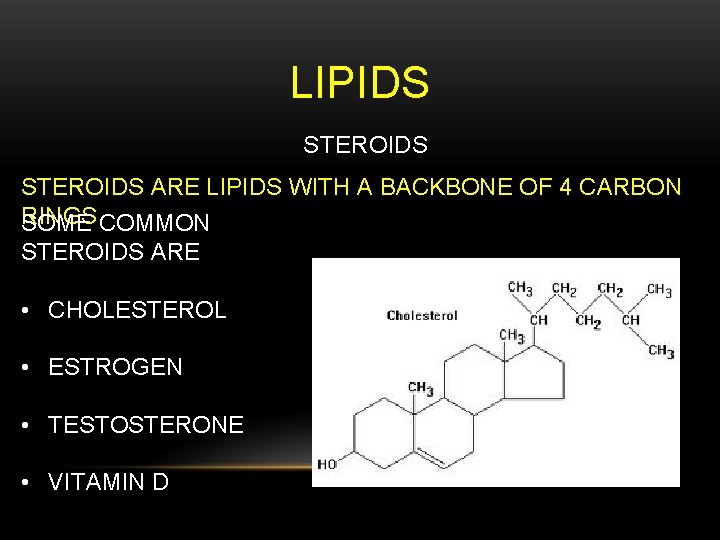
LIPIDS STEROIDS ARE LIPIDS WITH A BACKBONE OF 4 CARBON RINGS COMMON SOME STEROIDS ARE • CHOLESTEROL • ESTROGEN • TESTOSTERONE • VITAMIN D

NUCLEIC ACIDS MONOMER = NUCLEOTIDES ARE COMPOSED OF • 5 CARBON SUGAR (RIBOSE) • NITROGEN CONTAINING BASE (A, T, C, G) • PHOSPHATE GROUP ORGANISMS USE THE NUCLEOTIDE ATP FOR ____?
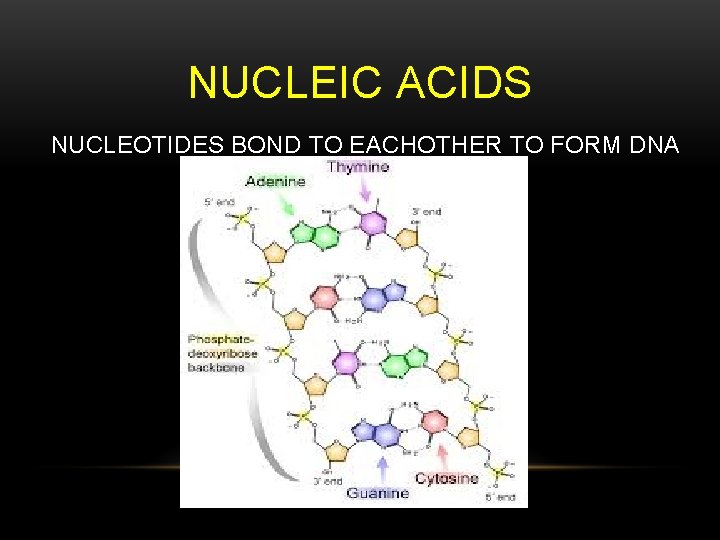
NUCLEIC ACIDS NUCLEOTIDES BOND TO EACHOTHER TO FORM DNA
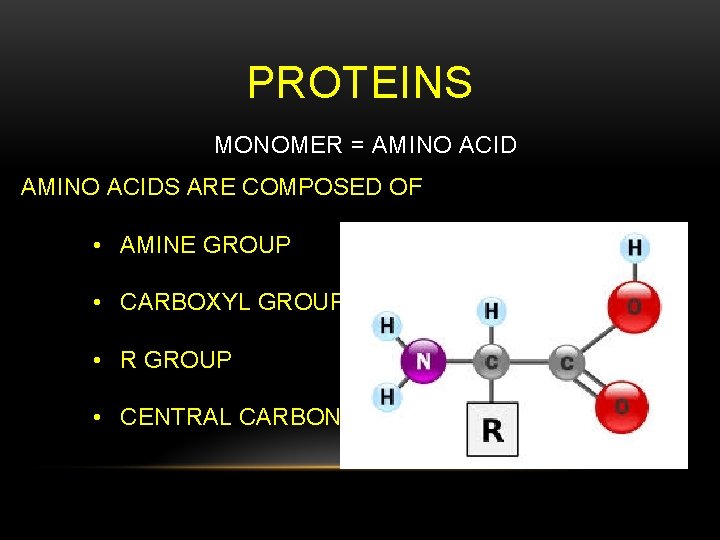
PROTEINS MONOMER = AMINO ACIDS ARE COMPOSED OF • AMINE GROUP • CARBOXYL GROUP • R GROUP • CENTRAL CARBON
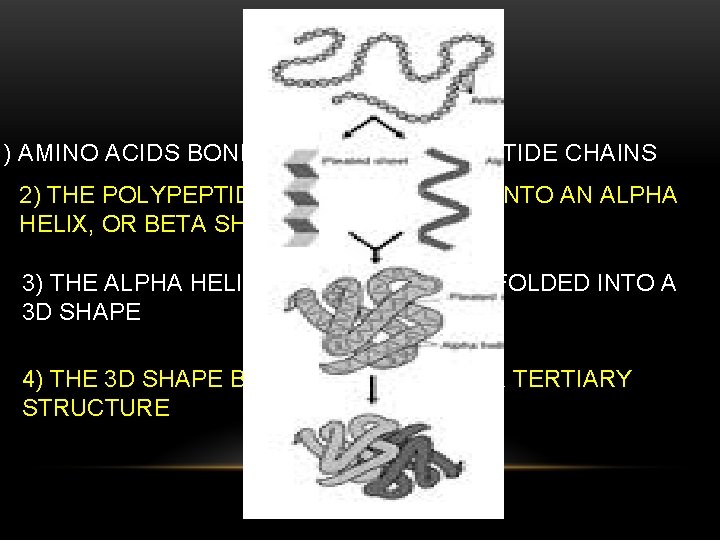
PROTEINS 1) AMINO ACIDS BOND TO MAKE POLY PEPTIDE CHAINS 2) THE POLYPEPTIDE CHAIN IS FORMED INTO AN ALPHA HELIX, OR BETA SHEET 3) THE ALPHA HELIX OR BETA SHEET IS FOLDED INTO A 3 D SHAPE 4) THE 3 D SHAPE BONDS WITH ANOTHER TERTIARY STRUCTURE
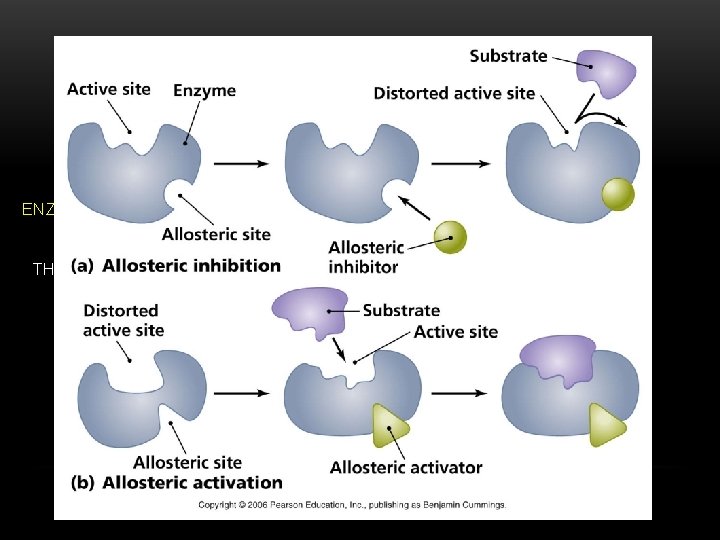
PROTEINS ENZYMES ARE PROTEINS THAT SPEED UP CHEMICAL REACTIONS, BY LOWERING THE ACTIVATION ENERGY THE INDUCED FIT MODEL EXPLAINES THAT THE EZYME FORMS TO THE SUBSTRATE

WATER ESSENTIAL FOR LIFE WATER IS POLAR DUE TO THE ELECTRONEGATIVITY OF THE OXYGEN ATOM IN THE MOLECULE

BECAUSE OF THE HYDROGEN BONDS WATER SPECIAL PROPERTIES • EXCELLENT SOLVENT • COHESION • ADHESION • HIGH HEAT OF VAPORIZATION • HIGH SPECIFIC HEAT • LOWER DENSITY AS A SOLID
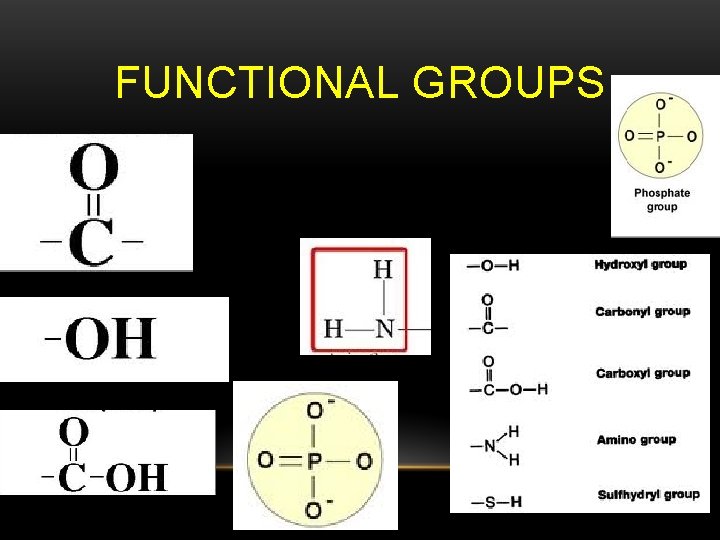
FUNCTIONAL GROUPS

• PROTEIN • BINGO! • WATER LIPID • PHOSPHOLIPID • NUCLEIC ACID • POLAR • CARBOHYDRATE • NONPOLAR ENZYME • • PRIMARY STRUCTURE • HYDROPHOBIC • SECONDARY STRUCTURE • HYDROPHILIC • TERTIARY STRUCTURE • AMINO ACID • QUATERNARY STRUCTURE • MONOSACCHARIDE • CARBON • NUCLEOTIDE • ACTIVE SITE • FATTY ACID • SUBSTRATE • DEHYDRATION SYNTHESIS • SATURATED FAT • POLYMER • UNSATURATED FAT • ADHESION • SOLVENT • COHESION • SOLUTE • HYDROGEN BOND • STEROIDS • PEPTIDE BOND • COFACTORS • COENZYME
- Slides: 21