BIOCHEMISTRY NOTES PT 3 FOUR MAIN TYPES OF
BIOCHEMISTRY NOTES PT. 3
FOUR MAIN TYPES OF ORGANIC MOLECULES THAT MAKE UP LIVING THINGS 1. CARBOHYDRATE 2. 3. 4. S LIPIDS (fats) PROTEINS NUCLEIC ACIDS • We call these four main types of carbon- based molecules MACROMOLECULES • These substances have certain MOLECULAR STRUCTURES & BIOCHEMICAL CHARACTERISTICS.

ORGANIC COMPOUNDS • Contain Carbon & Hydrogen • Necessary for life to exist • Macromolecules with a carbon backbone • These backbones can be 3 different forms
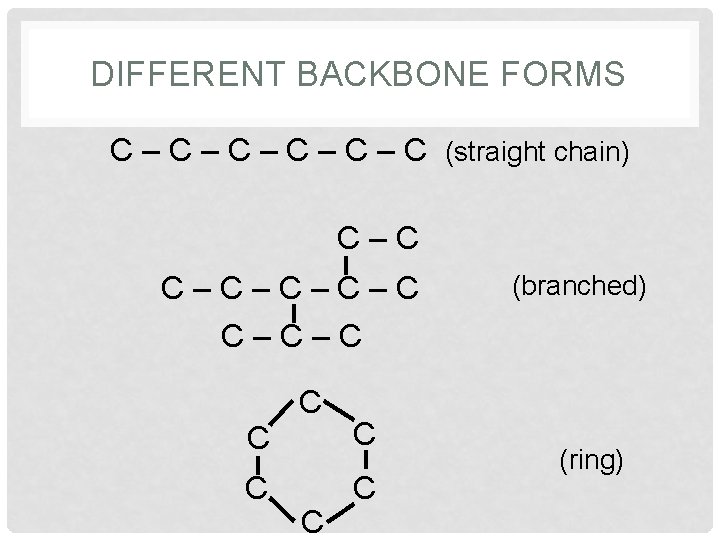
DIFFERENT BACKBONE FORMS C – C – C – C (straight chain) C–C–C–C–C C C C (branched) (ring)

BACKBONE FORM • The shape depends on the arrangement of the carbon atoms that make up the backbone • The shape defines the properties and function in living organisms.

CHNOPS • WHAT ARE THE ELEMENTS THAT ORGANIC MOLECULES? ? • Answer: • MAKE UP Organic molecules MUST be made of Carbon & Hydrogen. They CAN contain Nitrogen, Oxygen, Phosphorus & Sulfur.
MONOMERS & POLYMERS OF ORGANIC MOLECULES • Monomer – base unit or a building block of a polymer (macromolecules) • Mono means ONE • Poly means MANY • Polymer (macromolecules) – many monomers bonded together (long chain unit) • Examples: • Amino acids are the monomers (building blocks) of Proteins. • Monosaccharides are monomers of Carbohydrates
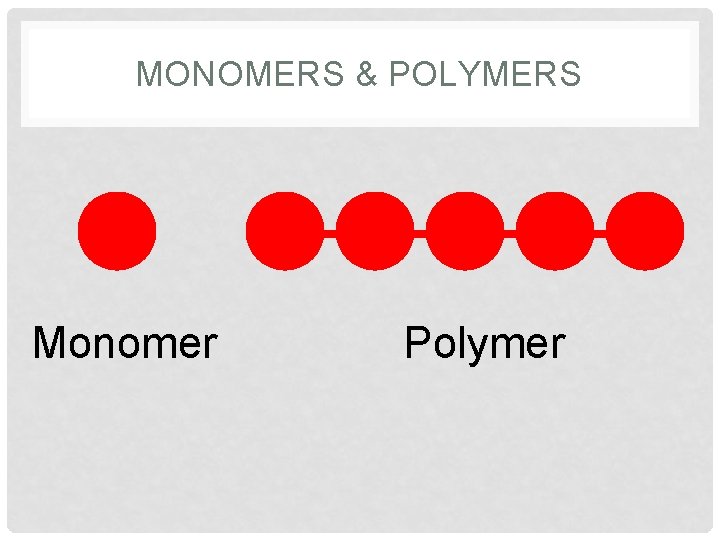
MONOMERS & POLYMERS Monomer Polymer
REMEMBER, REMEMBER… • Monomers (building blocks) covalently bond together to form a polymer (macromolecule)

ORGANIC MOLECULES OF LIVING THINGS • In addition to hydrogen and carbon they contain atoms of other elements • They tend to be large and complex

MACROMOLECULE 1: CARBOHYDRATES Simple Sugars Complex Carbs
MACROMOLECULE 1: CARBOHYDRATES Monosaccharides Polysaccharides
MACROMOLECULE 1: CARBOHYDRATES • Carbohydrates are used in our bodies to store potential energy & release energy when our bodies need to use it. • They are made of carbon, hydrogen & oxygen. • The simplest type of carbohydrate is a simple sugar called a monosaccharide. • Carbohydrates have a ratio of about 2 Hydrogens to 1 Carbon to 1 Oxygen. Glucose =C 6 H 12 O 6

WHAT IS YOUR FUNCTION? • The primary function of carbohydrates is for short-term energy storage (sugars are for Energy). • A secondary function is intermediate-term energy storage (as in starch for plants and glycogen for animals).
MONOSACCHARIDES VS. DISACCHARIDES VS. POLYSACCHARIDES • MONO = One • DI= Two • POLY= Many • Monosaccharides like glucose & fructose can combine together to form: • Disaccharides like sucrose which combine together to form: • Polysaccharides (polymer) like starch & cellulose. • WHICH IS BIGGER? A monosaccharide or a disaccharide or a polysaccharide?
MONOSACCHARIDES • Some carbohydrates are relatively small molecules, the most important to us is glucose which has 6 carbon atoms. • These simple sugars are called monosaccharides

DISACCHARIDES • Hooking two monosaccharides together forms a more complex sugar. • Compounds such as sucrose are called Disaccharides (two sugars). • Both monosaccharides and disaccharides are soluble in water.

POLYSACCHARIDES • Larger, more complex carbohydrates are formed by linking shorter units together to form long or very long sugar chains called Polysaccharides. • Because of their size, these are often times not soluble in water.
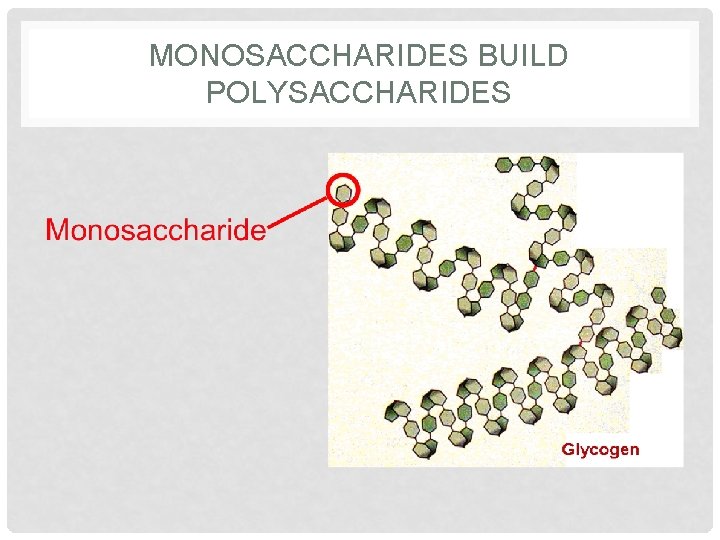
MONOSACCHARIDES BUILD POLYSACCHARIDES

BUT WAIT! THERE’S MORE • Many biologically important compounds such as starches and cellulose are Polysaccharides.

COMPLEX CARBOHYDRATE • Large polymers of sugars are called Carbohydrates. • The term Complex Carbohydrate, or sometimes even just Carbohydrate refers to long chains of sugars. • Three common types of complex carb's are: • Starch – used for long term energy storage in plants • Cellulose – used for plant cell wall structure • Glycogen – used for long term energy storage in humans

MONOMERS JOIN TOGETHER!. . . BUT HOW!? • The subunits (monomers) of the macromolecules (polymers) are covalently-bonded. • The covalent bonds between the subunits are always formed by a type of reaction • This reaction is called Dehydration Synthesis (making something while losing water – get it? LOSING WATER… DEHYDRATION!)

Dehydration Synthesis • When a water (H & OH) is removed from monosaccharides, a disaccharide can be formed. • When combining monosaccharides to form a disaccharide, there usually has to be a loss of an H and an OH. • This is also known as DEHYDRATION SYNTHESIS because a water molecule is removed.
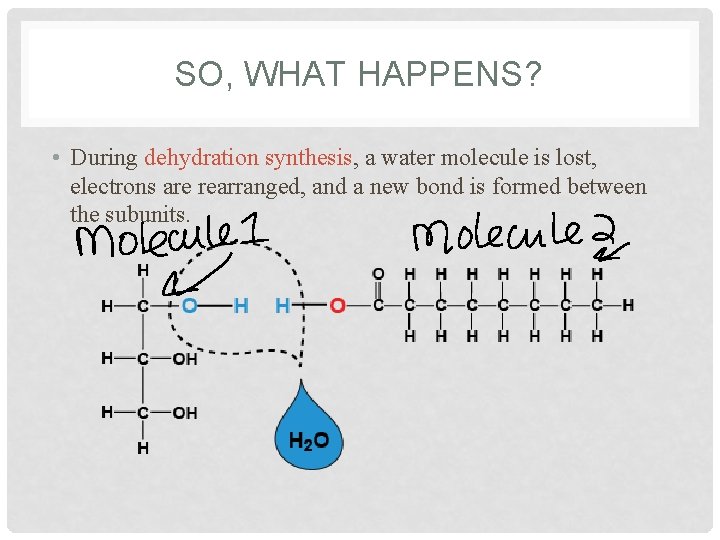
SO, WHAT HAPPENS? • During dehydration synthesis, a water molecule is lost, electrons are rearranged, and a new bond is formed between the subunits.
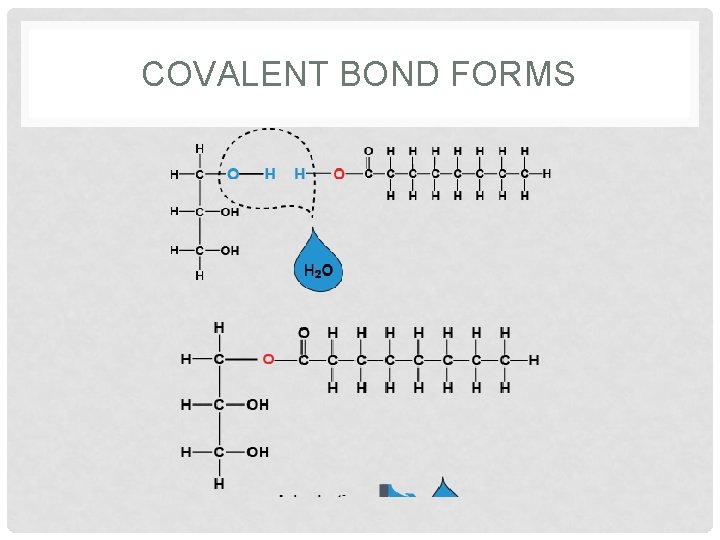
COVALENT BOND FORMS
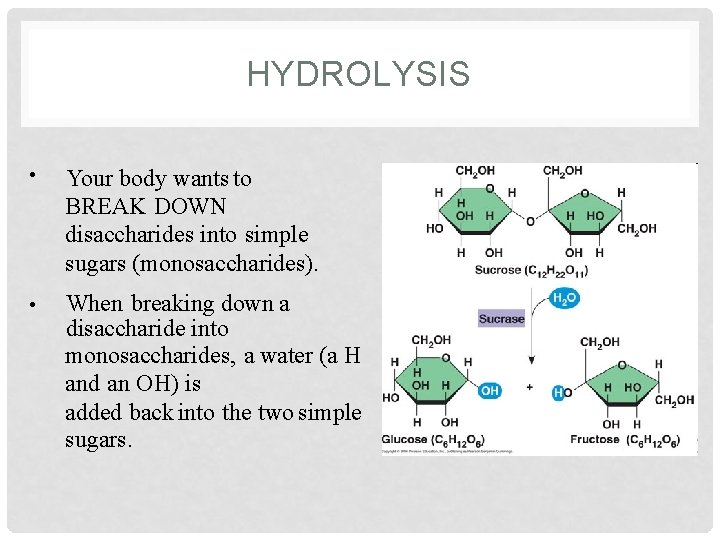
HYDROLYSIS • Your body wants to BREAK DOWN disaccharides into simple sugars (monosaccharides). • When breaking down a disaccharide into monosaccharides, a water (a H and an OH) is added back into the two simple sugars.
- Slides: 26