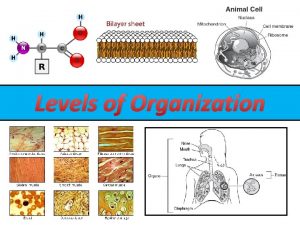
Biochemistry levels of organization Levels of Organization l

Biochemistry & levels of organization
Levels of Organization l Sub-atomic particles ……. put together make l Atoms……………. . . put together make l Molecules (monomers) …put together make l Macromolecules (polymers) …. . . . put together make l Organelles l Cells & Cell parts … put together make (smallest unit of life)
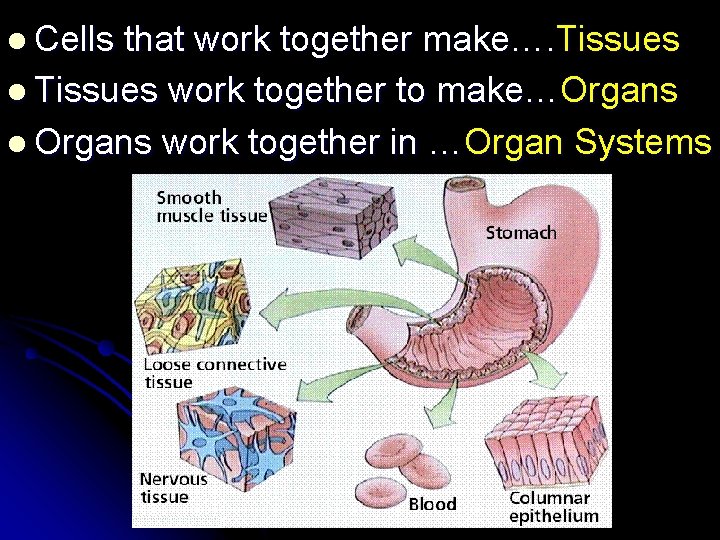
l Cells that work together make…. Tissues l Tissues work together to make…Organs l Organs work together in …Organ Systems

Sub-atomic Particles l Sub = smaller or under l Atomic = atom l ----------------------------- l Protons (+) l Neutrons (o) l Electrons (-)
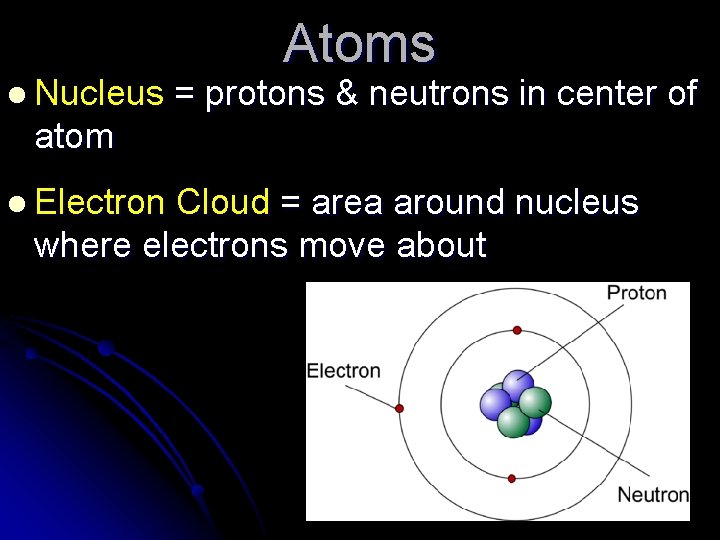
Atoms l Nucleus = protons & neutrons in center of atom l Electron Cloud = area around nucleus where electrons move about

Atoms l The number of protons in an atom determines the Element l The most common elements in living things are: l C H N O P S

Carbon l Hydrogen l Nitrogen l Oxygen l Phosphorous l Sulfur l Elements we need in only very small amounts are called l Trace elements l
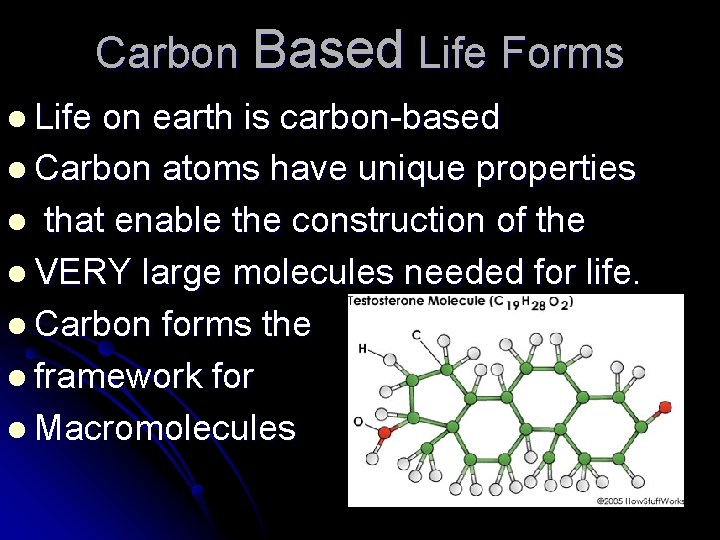
Carbon Based Life Forms l Life on earth is carbon-based l Carbon atoms have unique properties l that enable the construction of the l VERY large molecules needed for life. l Carbon forms the l framework for l Macromolecules
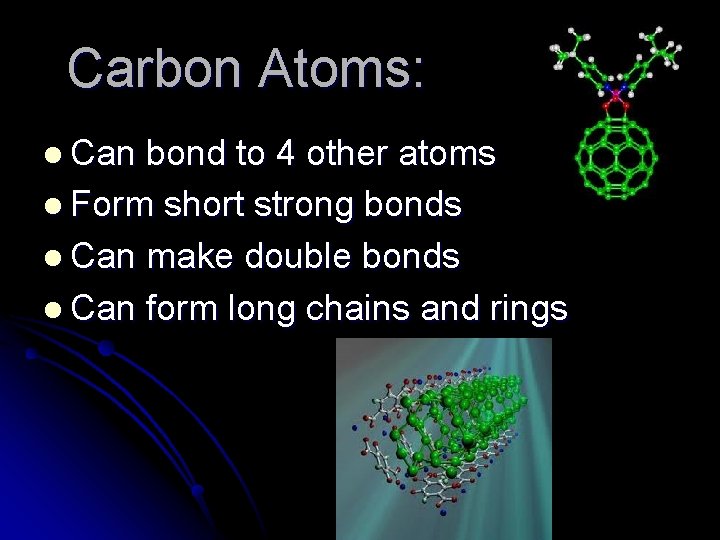
Carbon Atoms: l Can bond to 4 other atoms l Form short strong bonds l Can make double bonds l Can form long chains and rings

Monomers l Single molecules that make up all living things 1) monosaccharides = single sugars l 2) glycerol – alcohol used to connect things l 3) fatty acids – make l 4) amino acids - make l 5) Nucleotides – found in l - make l

1) Monosaccharides A) are carbohydrates = made of only C, H, O l carbon + water l B) examples : l 1) glucose – made by photosynthesis l 2) fructose – fruit sugar l C) 2 bound together make a disaccharide l example disaccharide = sucrose l D) several bound together = oligosaccharide l E) many can bond together = polysaccharide l (macromolecule) l
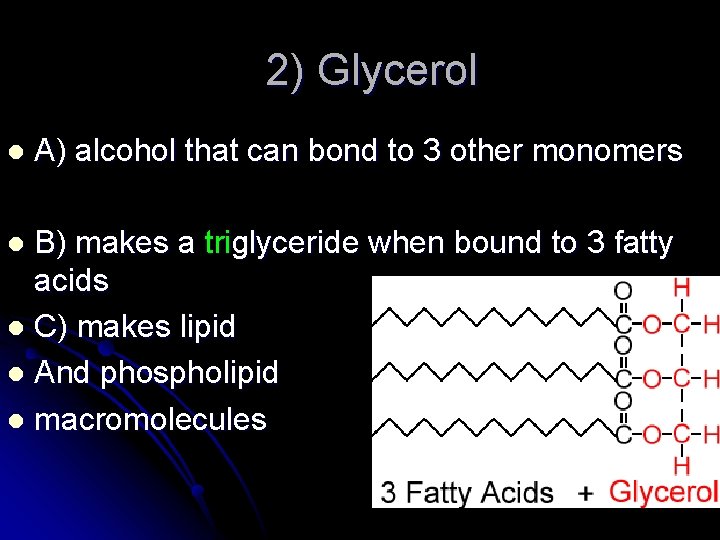
2) Glycerol l A) alcohol that can bond to 3 other monomers B) makes a triglyceride when bound to 3 fatty acids l C) makes lipid l And phospholipid l macromolecules l

3) Fatty Acids A) acid followed by a Hydrocarbon chain l (chain of H & C) l HC chains are hydrophobic l (water fearing) don’t mix w/ H 2 O l B) straight chains are saturated fatty acids l C) bent chains are unsaturated fatty acids l D) make lipids and l Phospholipid macromolecules l

E) Fatty Acid Nutrition Facts Fatty acid saturated Good in reasonable amounts Solid animal fats Butter, steak fat, lard, bacon grease unsaturated Best Liquid oils Vegetable oil, fish oil Partially hydrogenated BAD Soft solid oil = trans fats Most margarine, Crisco, some frying oil Omega 3 Good bsc we don’t get enough of it oil Fish, seafood, olive oil, walnuts, grass fed beef

World's Healthiest Foods rich in omega-3 fats Food. Cals. DRI/DV Flaxseeds 75133% Walnuts 196113% Sardines 18961% Salmon 15855% Beef 17546% Soybeans 29843% Tofu 16428% Shrimp 13514% Brussels Sprouts 5611% Cauliflower 299%
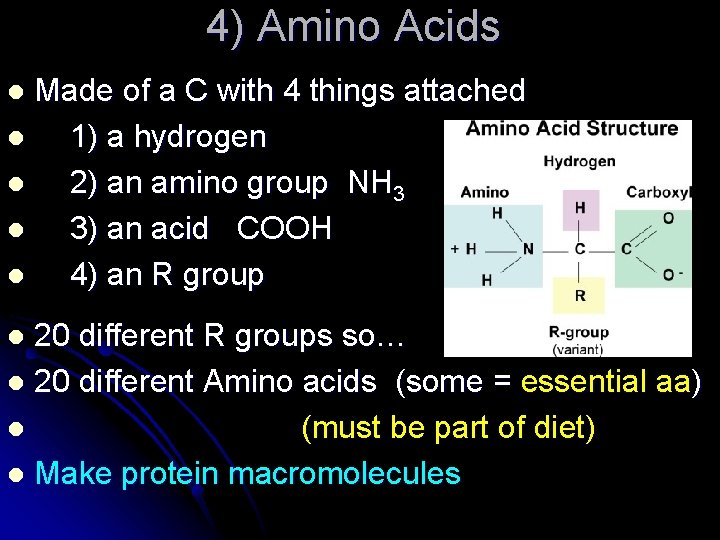
4) Amino Acids Made of a C with 4 things attached l 1) a hydrogen l 2) an amino group NH 3 l 3) an acid COOH l 4) an R group l 20 different R groups so… l 20 different Amino acids (some = essential aa) l (must be part of diet) l Make protein macromolecules l

5) Nucleotides l The largest monomer…actually includes a monosaccharide l Made of 3 parts l 1) one 5 carbon sugar l 2) phosphate 3) nitrogen containing base l
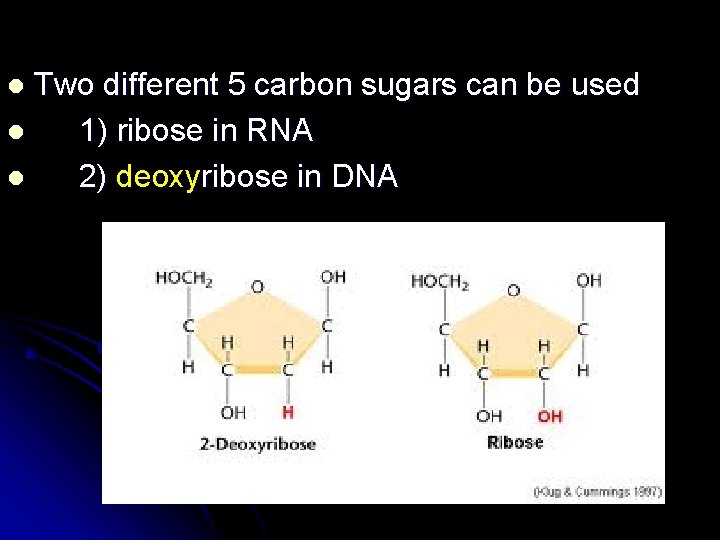
Two different 5 carbon sugars can be used l 1) ribose in RNA l 2) deoxyribose in DNA l
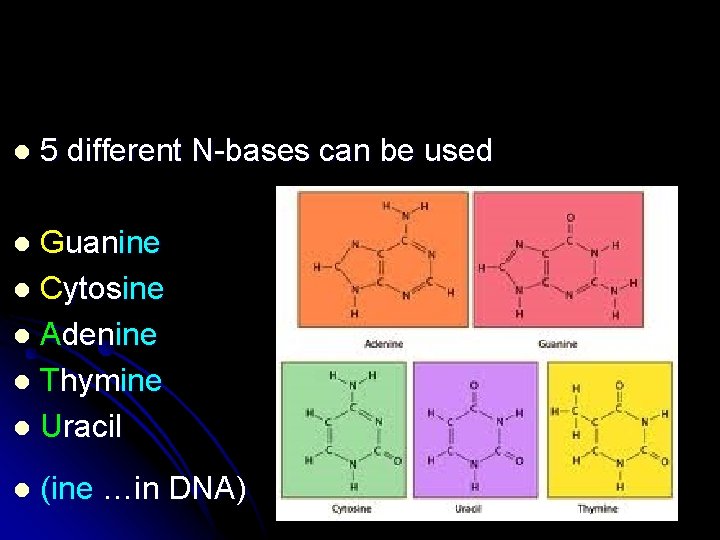
l 5 different N-bases can be used Guanine l Cytosine l Adenine l Thymine l Uracil l l (ine …in DNA)
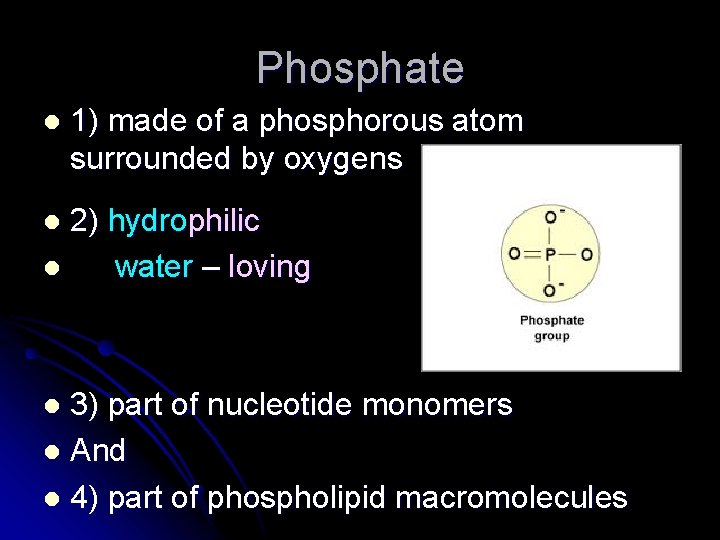
Phosphate l 1) made of a phosphorous atom surrounded by oxygens 2) hydrophilic l water – loving l 3) part of nucleotide monomers l And l 4) part of phospholipid macromolecules l
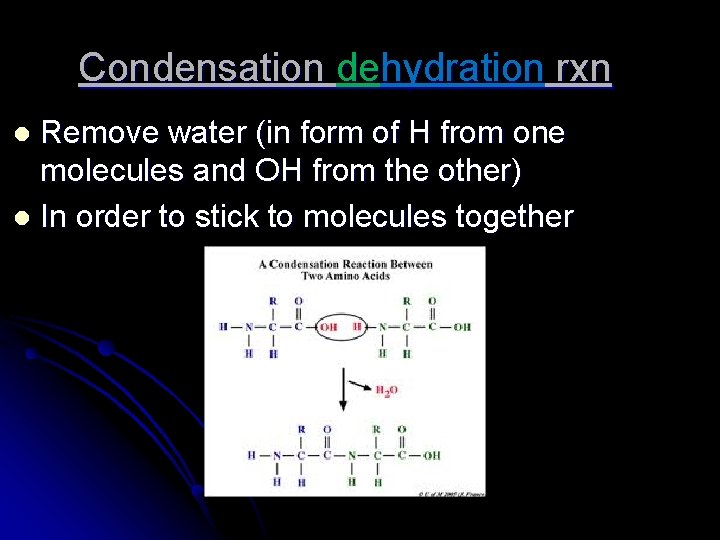
Condensation dehydration rxn Remove water (in form of H from one molecules and OH from the other) l In order to stick to molecules together l
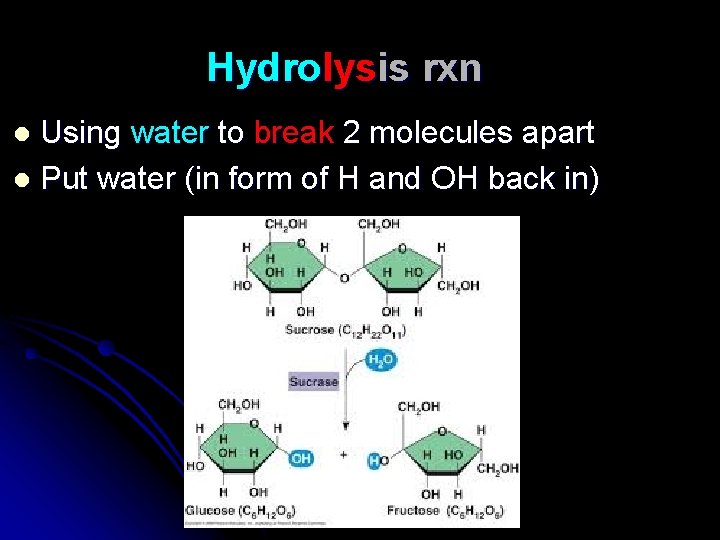
Hydrolysis rxn Using water to break 2 molecules apart l Put water (in form of H and OH back in) l
Polymers = Macromolecules Monomers bonded together to make l polymers l l Chemical reactions that bond monomers together called… Condensation dehydration reactions l (bring together) (remove H 2 O) l
5 Macromolecules l 1) polysaccharides l 2) Lipids l 3) phospholipids l 4) proteins l 5) nucleic acids
1) Polysaccharides l Made of many single sugars (carbohydrates) 1) starch – how plants store glucose l 2) glycogen – how animals store glucose l 3) cellulose – makes plant cell walls & l is the fiber in our diet l (we can’t digest it) l All 3 are made of chains of glucose l

2) Lipids Made of fatty acids & glycerol l Hydrophobic l 1) fats – solid at room temp. (saturated f. a. ) l store energy in animals l 2) oils – liquid at room temp. (unsaturated f. a. ) l store energy in plants l l 3) waxes – used to waterproof l 4) steroids – hormones help w/ homeostasis
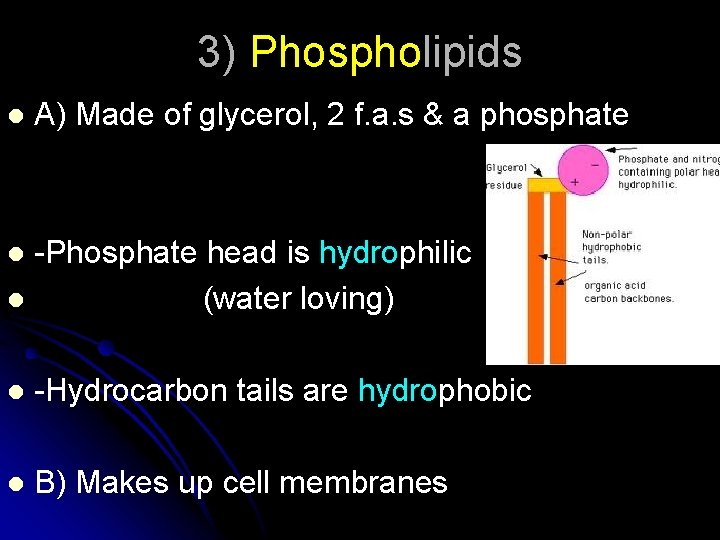
3) Phospholipids l A) Made of glycerol, 2 f. a. s & a phosphate -Phosphate head is hydrophilic l (water loving) l l -Hydrocarbon tails are hydrophobic l B) Makes up cell membranes
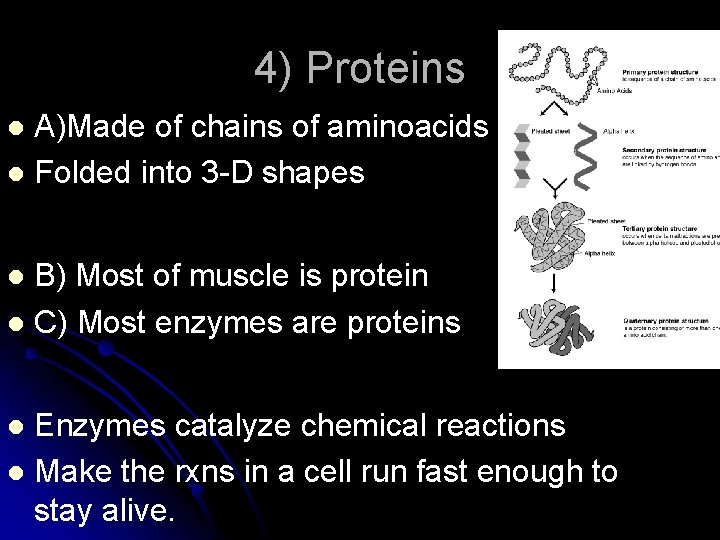
4) Proteins A)Made of chains of aminoacids l Folded into 3 -D shapes l B) Most of muscle is protein l C) Most enzymes are proteins l Enzymes catalyze chemical reactions l Make the rxns in a cell run fast enough to stay alive. l
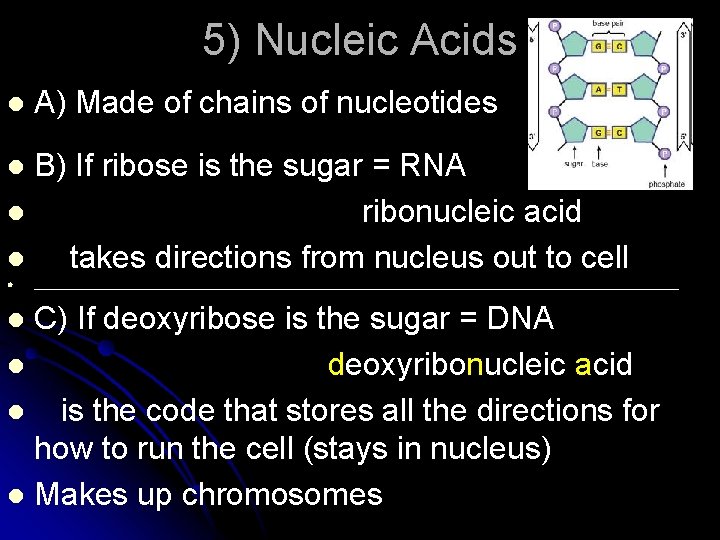
5) Nucleic Acids l A) Made of chains of nucleotides B) If ribose is the sugar = RNA l ribonucleic acid l takes directions from nucleus out to cell l l __________________________________________________________ C) If deoxyribose is the sugar = DNA l deoxyribonucleic acid l is the code that stores all the directions for how to run the cell (stays in nucleus) l Makes up chromosomes l
- Slides: 29