Biochemistry Gluconeogenesis TCA PPP Robyn Lincoln 2 nd

Biochemistry Gluconeogenesis, TCA, PPP Robyn Lincoln 2 nd Year Pharmacy Student Robyn. lincoln@bison. howard. edu 678 -234 -6617

METABOLIC PATHWAYS 1. Glycolysis 2. Gluconeogenesis 3. Pentose Phosphate Pathway In animals and vascular plants, glucose has three major fates: 1. It may be stored (as a polysaccharide or as sucrose); 2. Oxidized to a three-carbon compound (pyruvate) via glycolysis to provide ATP and metabolic intermediates; 3. Or oxidized via the pentose phosphate (phosphogluconate) pathway to yield ribose 5 -phosphate for nucleic acid synthesis and NADPH for reductive biosynthetic processes


1. GLUCONEOGENESIS - Comparison between Glycolysis & Gluconeogenesis - Regulation of Gluconeogenesis
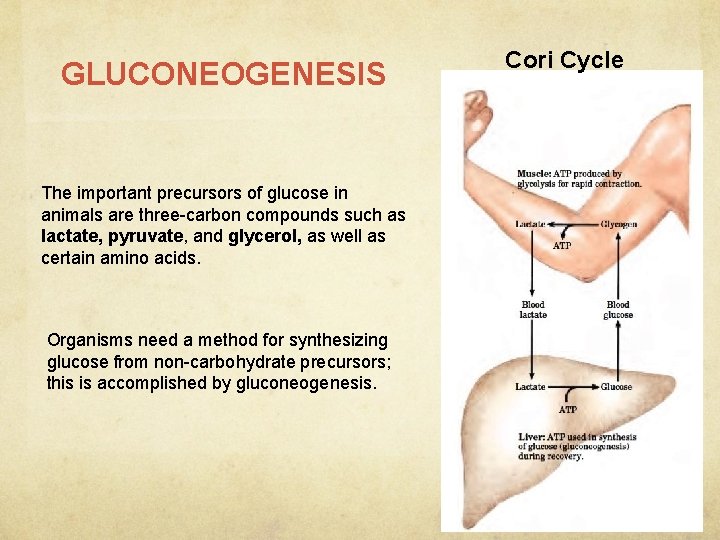
GLUCONEOGENESIS The important precursors of glucose in animals are three-carbon compounds such as lactate, pyruvate, and glycerol, as well as certain amino acids. Organisms need a method for synthesizing glucose from non-carbohydrate precursors; this is accomplished by gluconeogenesis. Cori Cycle
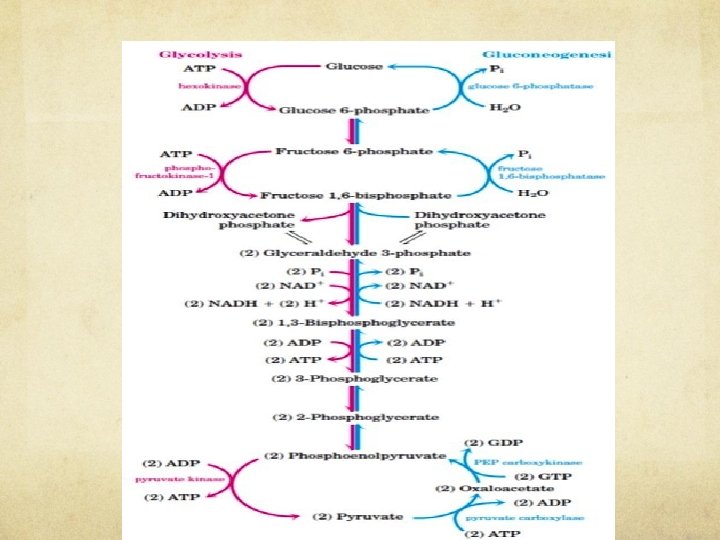
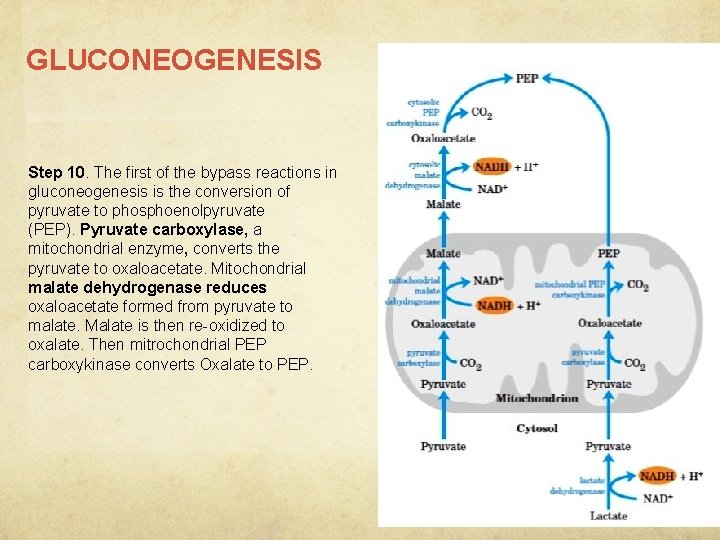
GLUCONEOGENESIS Step 10. The first of the bypass reactions in gluconeogenesis is the conversion of pyruvate to phosphoenolpyruvate (PEP). Pyruvate carboxylase, a mitochondrial enzyme, converts the pyruvate to oxaloacetate. Mitochondrial malate dehydrogenase reduces oxaloacetate formed from pyruvate to malate. Malate is then re-oxidized to oxalate. Then mitrochondrial PEP carboxykinase converts Oxalate to PEP.
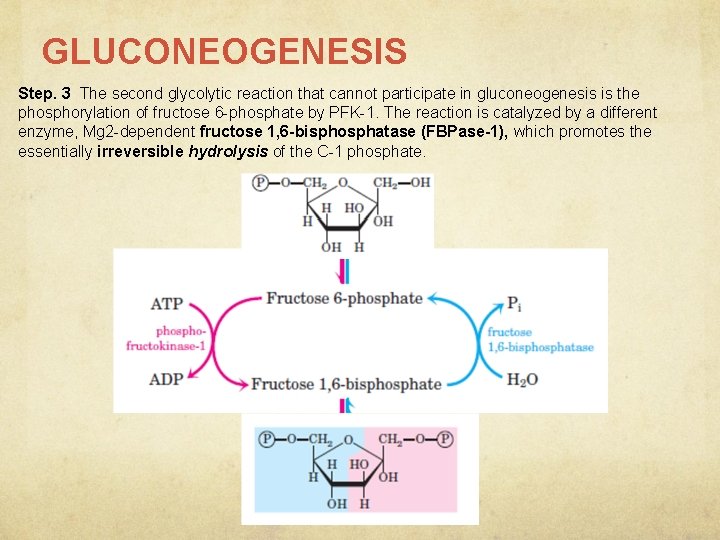
GLUCONEOGENESIS Step. 3 The second glycolytic reaction that cannot participate in gluconeogenesis is the phosphorylation of fructose 6 -phosphate by PFK-1. The reaction is catalyzed by a different enzyme, Mg 2 -dependent fructose 1, 6 -bisphosphatase (FBPase-1), which promotes the essentially irreversible hydrolysis of the C-1 phosphate.
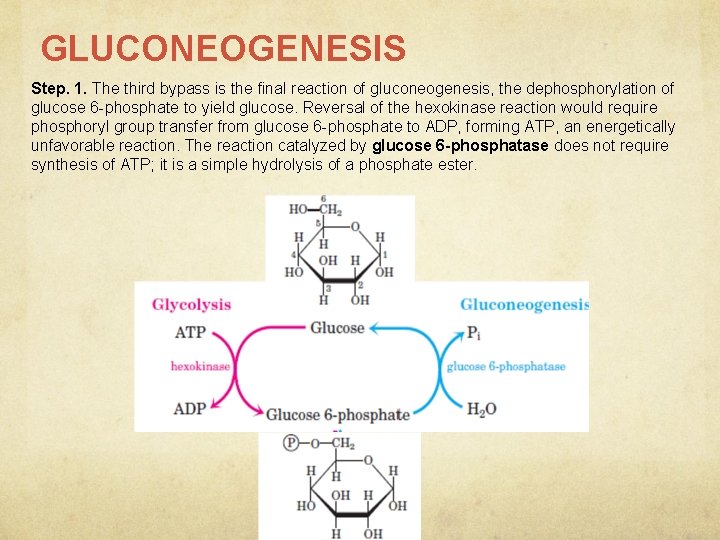
GLUCONEOGENESIS Step. 1. The third bypass is the final reaction of gluconeogenesis, the dephosphorylation of glucose 6 -phosphate to yield glucose. Reversal of the hexokinase reaction would require phosphoryl group transfer from glucose 6 -phosphate to ADP, forming ATP, an energetically unfavorable reaction. The reaction catalyzed by glucose 6 -phosphatase does not require synthesis of ATP; it is a simple hydrolysis of a phosphate ester.
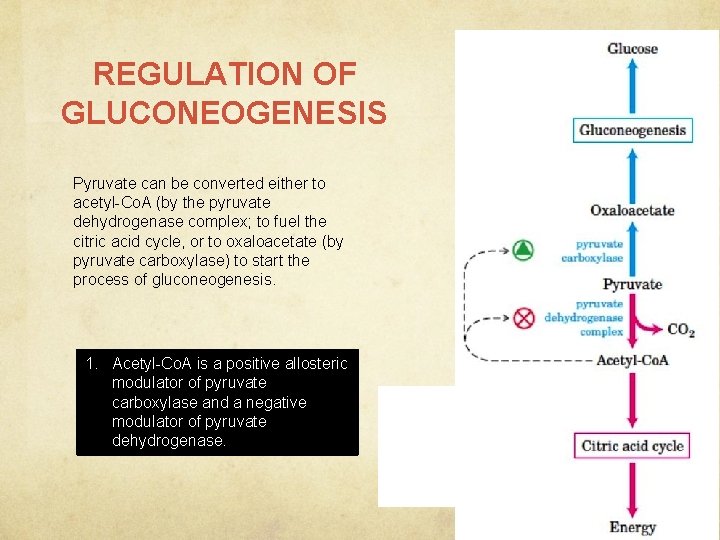
REGULATION OF GLUCONEOGENESIS Pyruvate can be converted either to acetyl-Co. A (by the pyruvate dehydrogenase complex; to fuel the citric acid cycle, or to oxaloacetate (by pyruvate carboxylase) to start the process of gluconeogenesis. 1. Acetyl-Co. A is a positive allosteric modulator of pyruvate carboxylase and a negative modulator of pyruvate dehydrogenase.
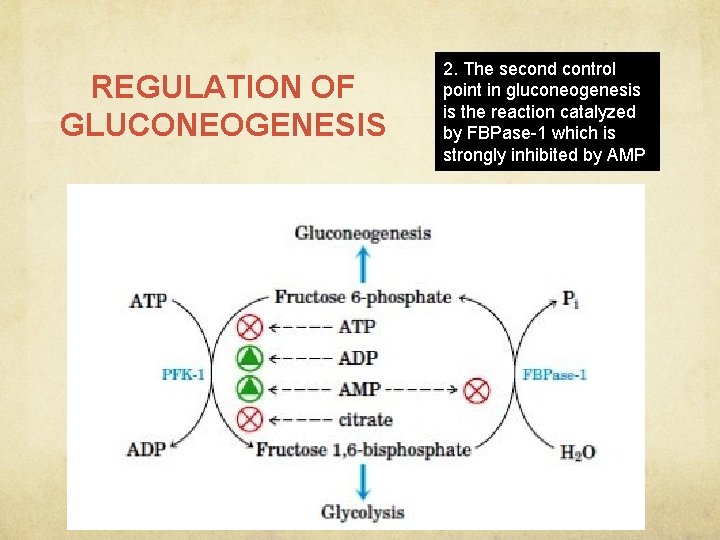
REGULATION OF GLUCONEOGENESIS 2. The second control point in gluconeogenesis is the reaction catalyzed by FBPase-1 which is strongly inhibited by AMP
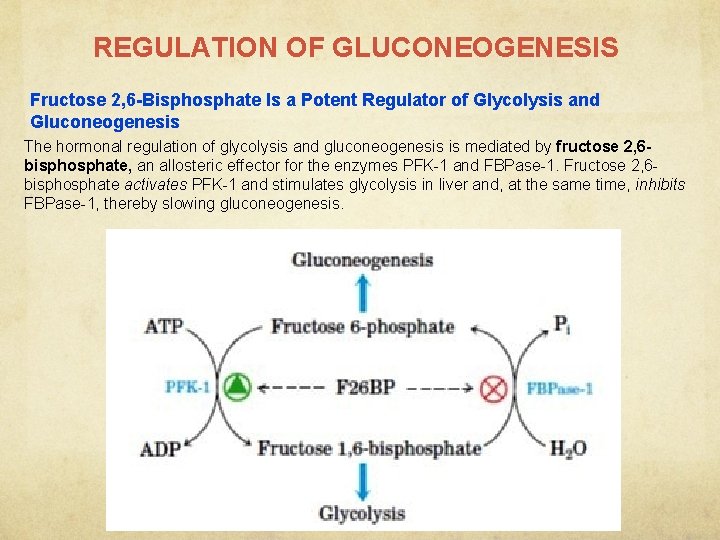
REGULATION OF GLUCONEOGENESIS Fructose 2, 6 -Bisphosphate Is a Potent Regulator of Glycolysis and Gluconeogenesis The hormonal regulation of glycolysis and gluconeogenesis is mediated by fructose 2, 6 bisphosphate, an allosteric effector for the enzymes PFK-1 and FBPase-1. Fructose 2, 6 bisphosphate activates PFK-1 and stimulates glycolysis in liver and, at the same time, inhibits FBPase-1, thereby slowing gluconeogenesis.
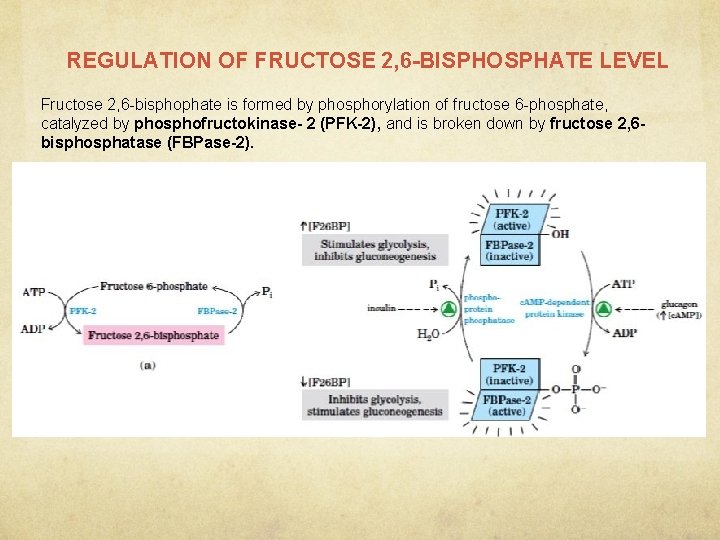
REGULATION OF FRUCTOSE 2, 6 -BISPHOSPHATE LEVEL Fructose 2, 6 -bisphophate is formed by phosphorylation of fructose 6 -phosphate, catalyzed by phosphofructokinase- 2 (PFK-2), and is broken down by fructose 2, 6 bisphosphatase (FBPase-2).

WHEN IS GLUCONEOGENESIS HAPPENING? ● Gluconeogenesis creates glucose from non-carbohydrate precursors ● GNG occurs when there are no sources of sugar o No sugar in the blood o All glycogen (glucose polymer, stored in liver/muscles) has been used up o Body can use waste materials from other cycles/other food metabolism (lipids mostly) ● In fasting or starvation states, all generated glucose is first transported to the brain

2. TCA CYCLE - Products obtained from citric acid cycle
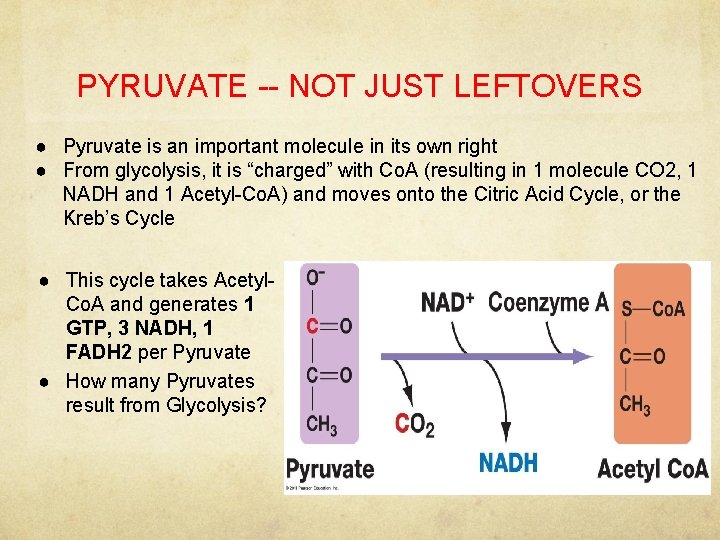
PYRUVATE -- NOT JUST LEFTOVERS ● Pyruvate is an important molecule in its own right ● From glycolysis, it is “charged” with Co. A (resulting in 1 molecule CO 2, 1 NADH and 1 Acetyl-Co. A) and moves onto the Citric Acid Cycle, or the Kreb’s Cycle ● This cycle takes Acetyl. Co. A and generates 1 GTP, 3 NADH, 1 FADH 2 per Pyruvate ● How many Pyruvates result from Glycolysis?
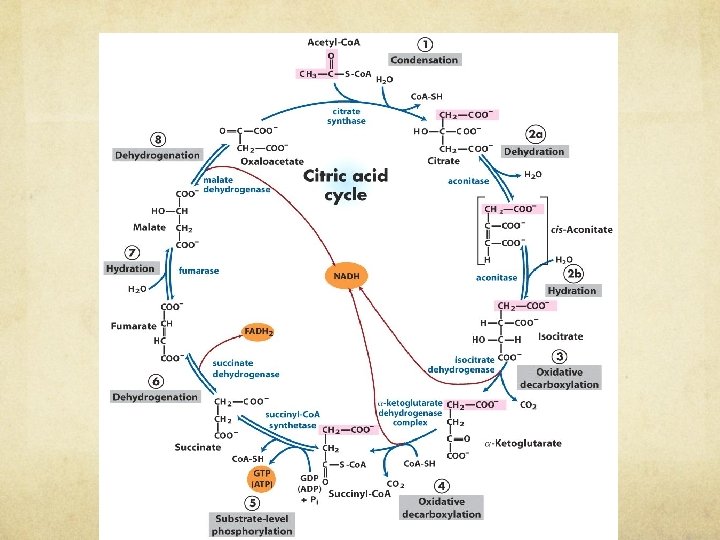

CITRIC ACID CYCLE ● Acetyl-Co. A (2 C) joined with Oxaloacetate (4 C) to make Citrate (6 C) ● Carbons are swapped around and bonds are oxidized and reduced, until… ● The two extra C’s are chopped off as 2 CO 2 ● Another Acetyl-Co. A is added, cycle resumes Each molecule of glucose produces how many pyruvates? o How many times must the TCA turn to process one glucose molecule? o
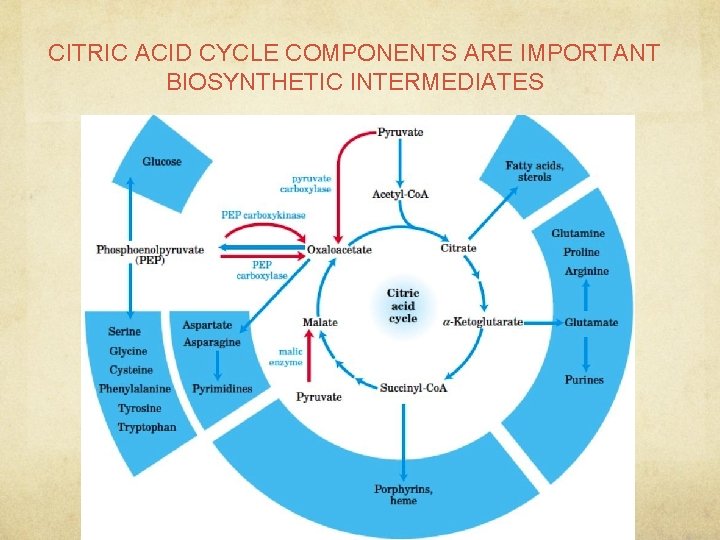
CITRIC ACID CYCLE COMPONENTS ARE IMPORTANT BIOSYNTHETIC INTERMEDIATES

What do we need to know from TCA? ● TCA has 8 steps and plenty of intermediates o These 8 -6 carbon molecules are used for many structures and functions in the body § Ex: alpha-ketoglutarate is important for the synthesis and maintenance of amino acid pathway; it is a nitrogen shuttle and supplies aminos for biosynth of AA o However, while it is convenient to snatch pre-made molecules from an ongoing cycle, the cycle still needs to keep enough intermediates to function! § Anaplerosis is the process of replenishing TCA cycle intermediates that have been extracted for biosynthesis § What does this word look like? (HINT: ANA) o Biochemical pathways MUST BE BALANCED or else someone is going to run out of energy and/or necessary molecules

TCA = END OF THE ROAD ● Glucose goes through glycolysis then the end-products, 2 pyruvate, go through the TCA cycle ● What do we get out of this?
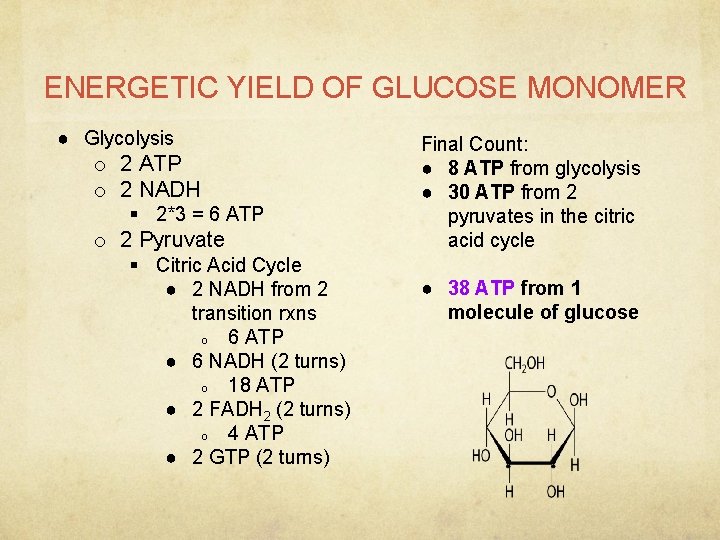
ENERGETIC YIELD OF GLUCOSE MONOMER ● Glycolysis o 2 ATP o 2 NADH § 2*3 = 6 ATP o 2 Pyruvate § Citric Acid Cycle ● 2 NADH from 2 transition rxns o 6 ATP ● 6 NADH (2 turns) o 18 ATP ● 2 FADH 2 (2 turns) o 4 ATP ● 2 GTP (2 turns) Final Count: ● 8 ATP from glycolysis ● 30 ATP from 2 pyruvates in the citric acid cycle ● 38 ATP from 1 molecule of glucose

CITRIC ACID: INS AND OUTS ● What goes in: o o o Acetyl-Co. A H 2 O 3 NAD+ 1 FAD 1 GDP ● What comes out: ○ ○ 2 CO 2 3 NADH 1 FADH 2 1 GTP

Metabolic Cofactors ● You’ve seen NAD+/NADH floating around, right? o They carry H’s around to reduce and oxidize molecules for energy o Also carry those electrons/H’s to the Ox. Phos Pathway, which we’ll talk about next class o FADH 2 functions similarly, can “extract” 2 H o However, NAD+ has a cousin… ● NADH is a catabolic (energy-generating) cofactor o Whenever you see NADH in a pathway, you know the body is breaking down molecules for energy ● NADPH is an anabolic (biosynthetic) cofactor o Whenever you see NADPH in a pathway, you know that the body is conducting biosynthesis and some molecule is being made o A good example of an anabolic pathway is the PPP. . .

3. PENTOSE PHOSPHATE PATHWAY - Oxidative Phase - Non-Oxidative Phase
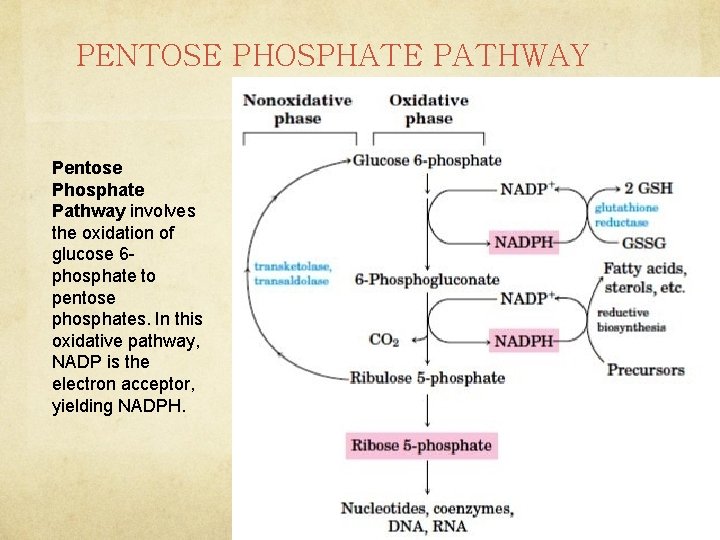
PENTOSE PHOSPHATE PATHWAY Pentose Phosphate Pathway involves the oxidation of glucose 6 phosphate to pentose phosphates. In this oxidative pathway, NADP is the electron acceptor, yielding NADPH.
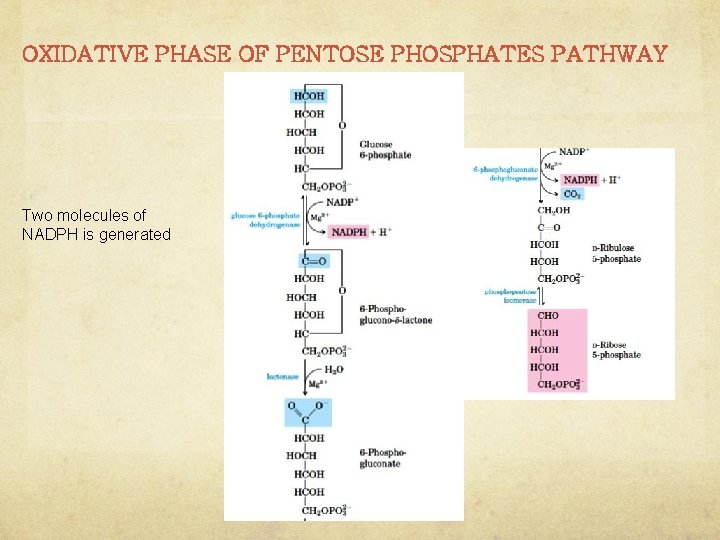
OXIDATIVE PHASE OF PENTOSE PHOSPHATES PATHWAY Two molecules of NADPH is generated
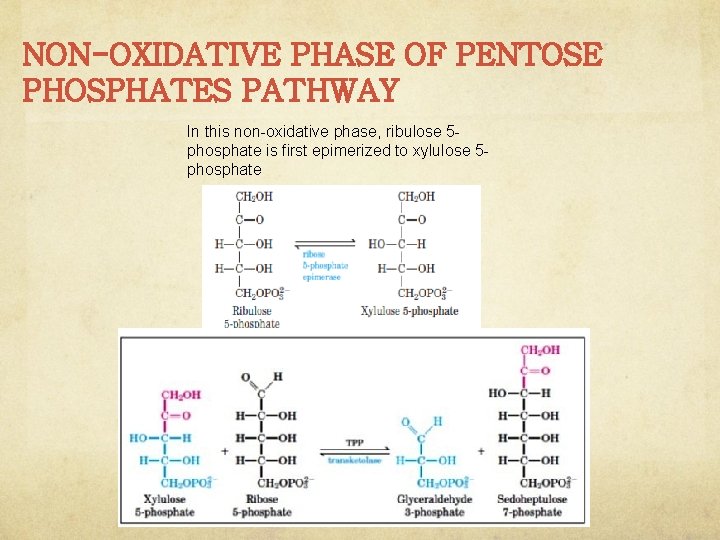
NON-OXIDATIVE PHASE OF PENTOSE PHOSPHATES PATHWAY In this non-oxidative phase, ribulose 5 phosphate is first epimerized to xylulose 5 phosphate
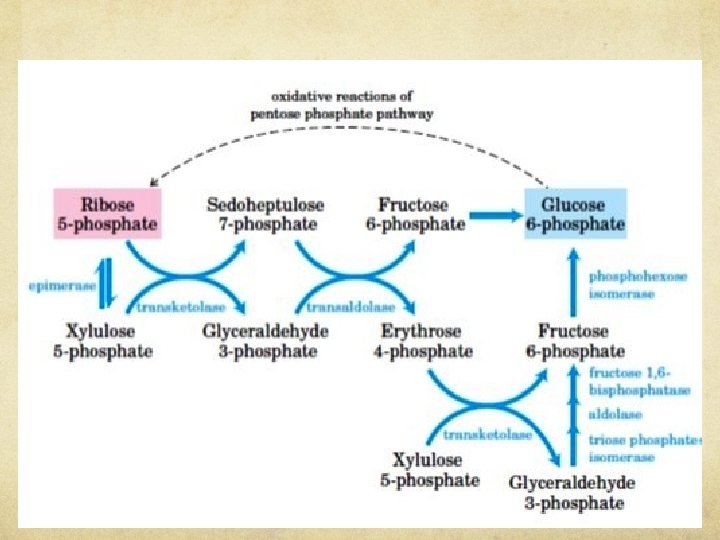
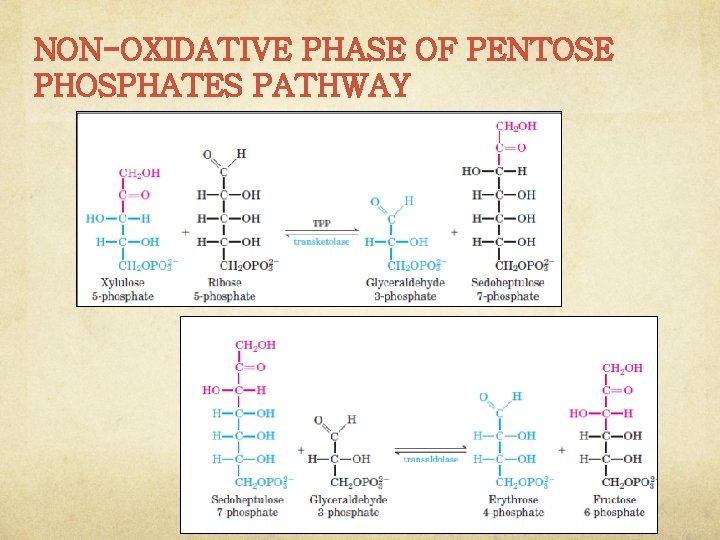
NON-OXIDATIVE PHASE OF PENTOSE PHOSPHATES PATHWAY
PRINCIPLES OF METABOLIC REGULATION How do cells to maintain a balance even in the face of outside perturbation?

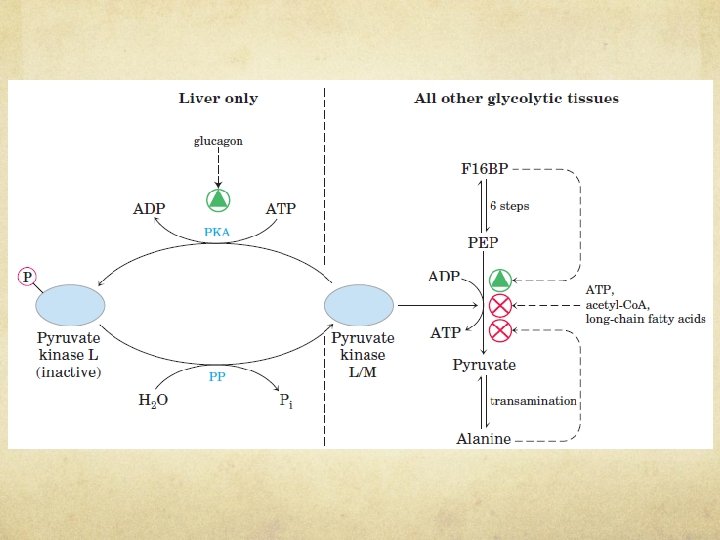
COORDINATED REGULATION OF GLYCOLYSIS AND GLUCONEOGENESIS Hexokinase Isozymes of Muscle and Liver Are Affected Differently by Their Product, Glucose 6 Phosphate Ø Hexokinase, which catalyzes the entry of free glucose into the glycolytic pathway, is a regulatory enzyme. Ø There are four isozymes (designated I to IV). Ø Hexokinase II has a high affinity for glucose Ø Muscle hexokinases I and II are allosteric ally inhibited by their product, glucose 6 phosphate, so whenever the cellular concentration of glucose 6 -phosphate rises above its normal level, these isozymes are temporarily and reversibly inhibited, bringing the rate of glucose 6 -phosphate formation into balance with the rate of its utilization and re-establishing the steady state. What mechanism does this sound like?
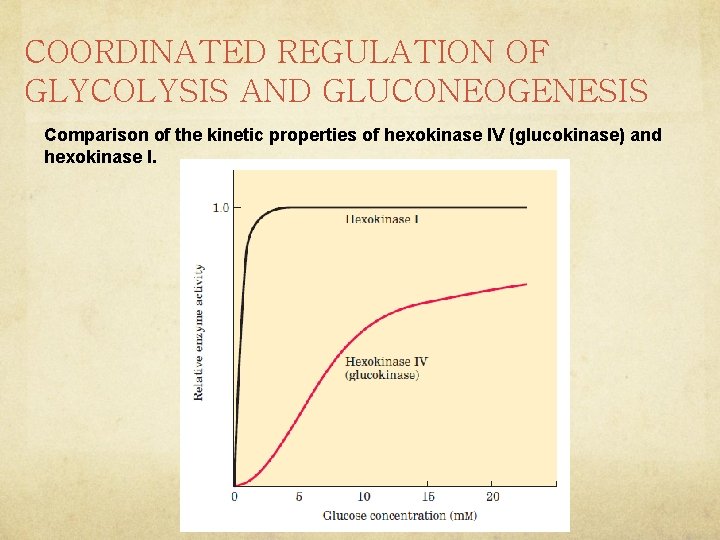
COORDINATED REGULATION OF GLYCOLYSIS AND GLUCONEOGENESIS Comparison of the kinetic properties of hexokinase IV (glucokinase) and hexokinase I.
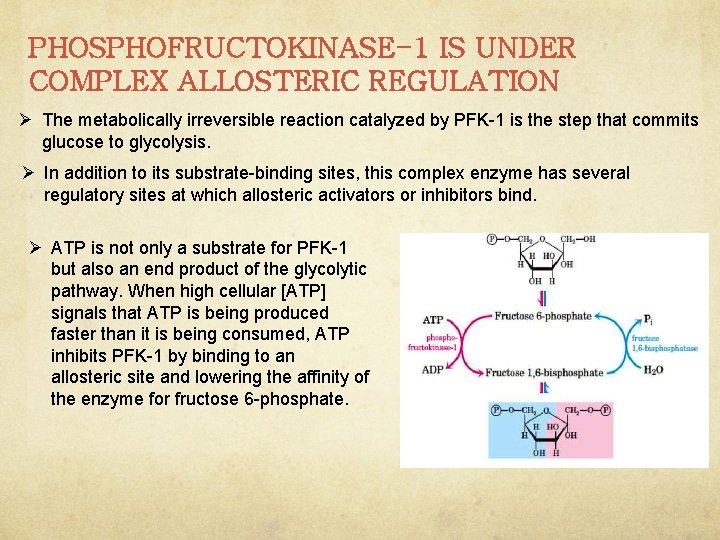
PHOSPHOFRUCTOKINASE-1 IS UNDER COMPLEX ALLOSTERIC REGULATION Ø The metabolically irreversible reaction catalyzed by PFK-1 is the step that commits glucose to glycolysis. Ø In addition to its substrate-binding sites, this complex enzyme has several regulatory sites at which allosteric activators or inhibitors bind. Ø ATP is not only a substrate for PFK-1 but also an end product of the glycolytic pathway. When high cellular [ATP] signals that ATP is being produced faster than it is being consumed, ATP inhibits PFK-1 by binding to an allosteric site and lowering the affinity of the enzyme for fructose 6 -phosphate.
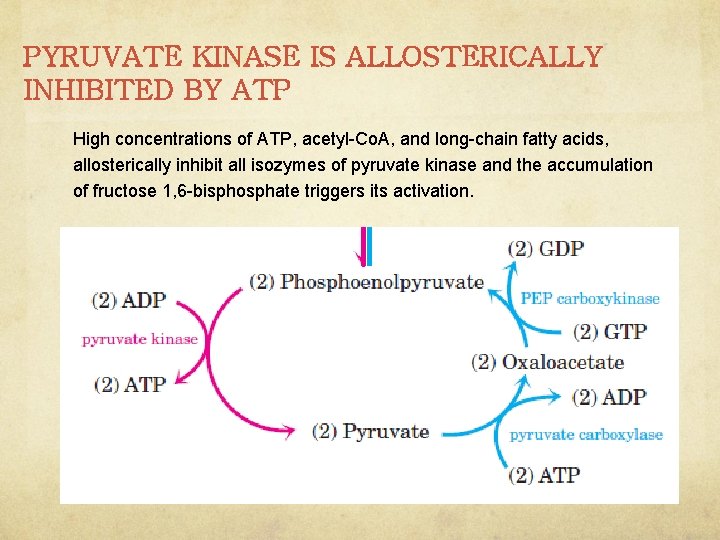
PYRUVATE KINASE IS ALLOSTERICALLY INHIBITED BY ATP High concentrations of ATP, acetyl-Co. A, and long-chain fatty acids, allosterically inhibit all isozymes of pyruvate kinase and the accumulation of fructose 1, 6 -bisphosphate triggers its activation.

Thank you!
- Slides: 38