BIOCHEMISTRY Fundamental Life Processes depend on the physical
BIOCHEMISTRY “Fundamental Life Processes depend on the physical structure and chemical activities of the cell. ” L 3 BIOLOGY CHAPTER 3
How are organisms structured to ensure efficiency and survival? To answer this question, you must know some chemistry…when we’ve completed this unit you should be able to l Describe significant similarities and differences in the basic structure of plant and animal cells. l Describe the general role of DNA and RNA in protein synthesis. l l Describe the general role of enzymes in metabolic cell processes. Explain the role of the cell membrane in supporting cell functions.

What is the structure of an atom? l l l Subatomic particles: Proton (+), neutron (neutral), electon (-) Atomic mass: Number of Protons AND Neutrons in the atom Atomic Number: Number of Protons in the atom (this number differs for each element!)

l How do we determine number of Electrons? l l Same as number of Protons in a neutral atom How do we determine number of Neutrons? l Atomic Mass - Atomic Number= # Neutrons
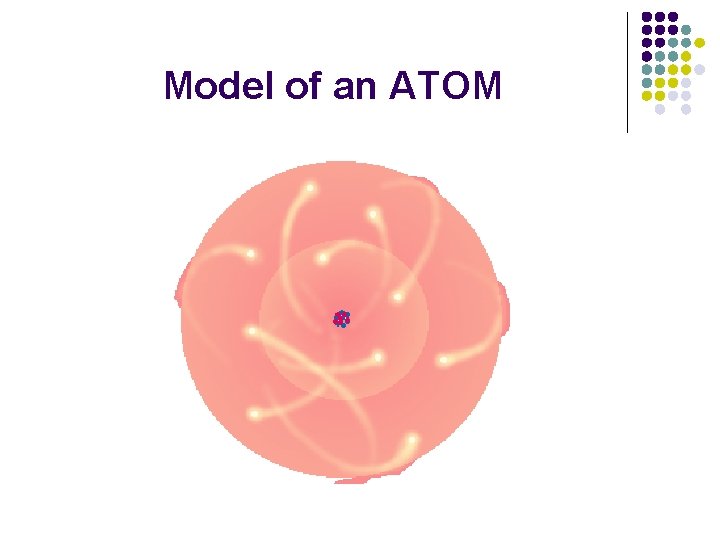
Model of an ATOM
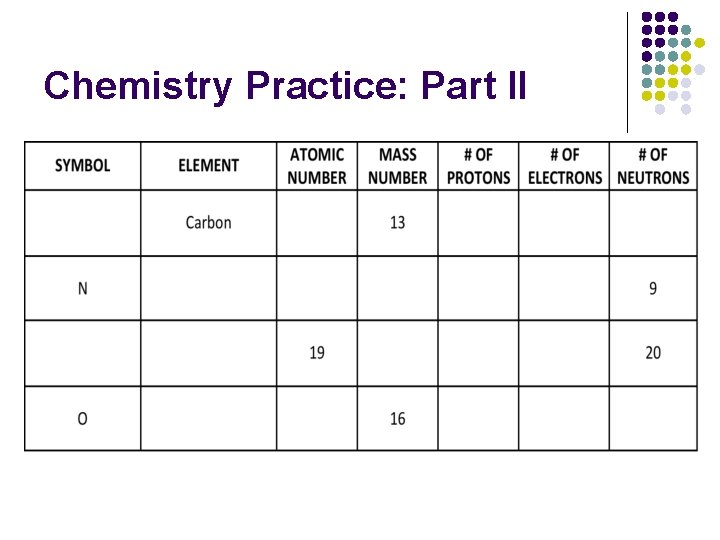
Chemistry Practice: Part II
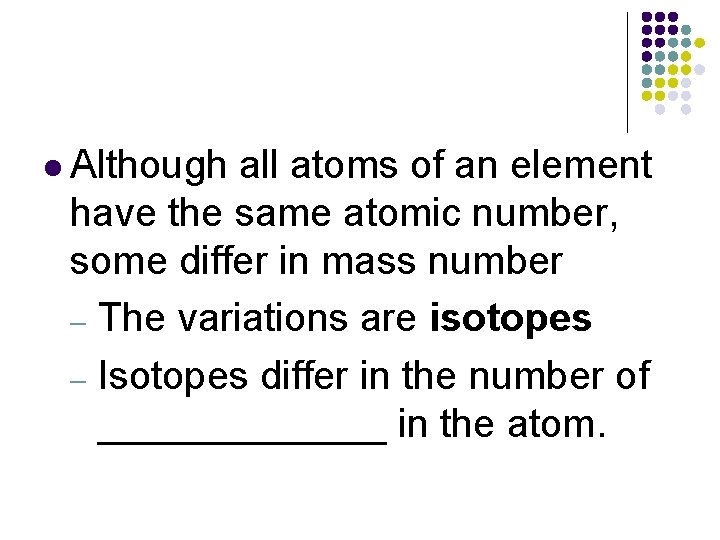
l Although all atoms of an element have the same atomic number, some differ in mass number – The variations are isotopes – Isotopes differ in the number of _______ in the atom.
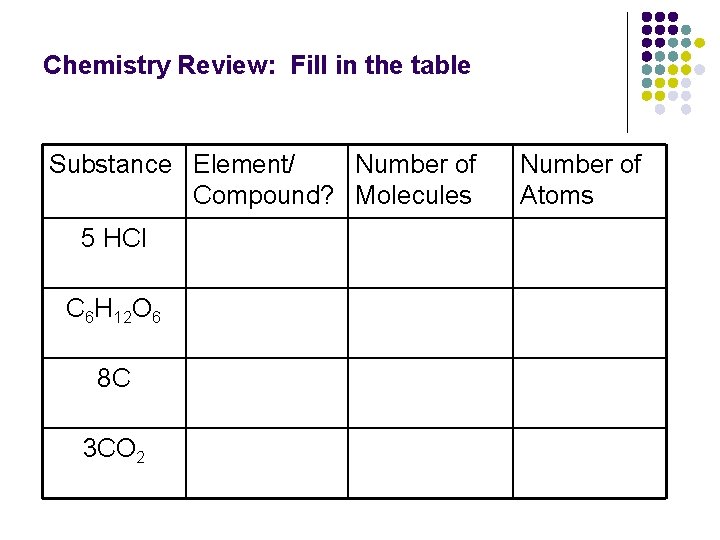
Chemistry Review: Fill in the table Substance Element/ Number of Compound? Molecules 5 HCl C 6 H 12 O 6 8 C 3 CO 2 Number of Atoms

Electron arrangement determines the chemical properties of an atom l l – – – Only electrons are involved in chemical activity Electrons occur in energy levels called electron shells Number of electrons in outermost shell determines chemical properties of atom First shell is full with two electrons Second and third hold up to eight electrons

Chemistry Practice: Part II l l Draw a hydrogen and helium atom. Label the protons, neutrons and electrons. Assume that they are neutral atoms and the common isotope. Draw an atom of Oxygen

Why do atoms form bonds? l Atoms want to fill their outer electron shells (Valence electrons) – To accomplish this, the atom can share, donate, or receive electrons – This results in attractions between atoms called chemical bonds

Chemistry Review: Part III l Atoms fill their valence shell by gaining/losing or sharing electrons. l When atoms gain/lose electrons the bonds are IONIC When atoms share electrons, the bonds are COVALENT l l An atom will form enough covalent bonds to fill the valence shell.

Ionic bonds are attractions between ions of opposite charge l Ion - an atom or molecule with an electrical charge resulting from gain or loss of electrons – l When an electron is lost, a positive charge results; when one is gained, a negative charge results Two ions with opposite charges attract each other – When the attraction holds the ions together, it is called an ionic bond

Covalent bonds form molecules when atoms share electrons l l A covalent bond results when atoms share outer-shell electrons – A molecule is formed when atoms are held together by covalent bonds Covalent bonds can involve equal sharing (nonpolar) or unequal sharing (polar) of the electrons.

Chemistry Practice: Part III l l Draw the valence electrons of C, H and O. How many covalent bonds does each need to fill the valence shell?
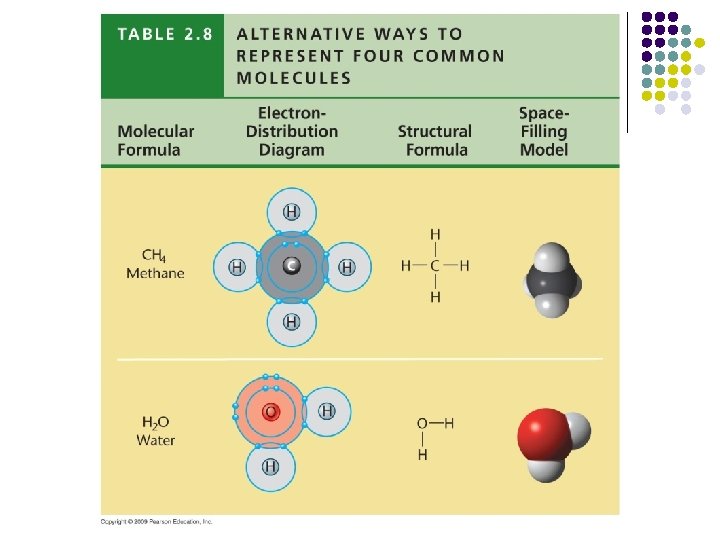
Chemistry Practice: Part III l l Valence electrons and covalent bond activity. Build: l l l CH 4 H 2 O CO 2 C 2 H 4 C 3 H 8 NH 3 HINT: Carbons like to bond together to form chains! HINT: Sometimes atoms share more than once (i. e. , form double/triple bonds)

Unequal electron sharing creates polar molecules l Atoms in a covalently bonded molecule continually compete for shared electrons – The attraction (pull) for shared electrons is called electronegativity – More electronegative atoms (like Oxygen!) pull harder

What favorite Polar molecule is ESSENTIAL for life? H 2 O!! Why? ? Because of its structure and properties (Remember: it has a gas, liquid & solid form)

What is the structure of H 2 O? l Water has atoms with different electronegativities, oxygen and hydrogen: – Oxygen attracts the shared electrons more strongly than hydrogen; – Shared electrons spend more time near oxygen which results in a polar covalent bond

Result: the oxygen atom has a slight negative charge and the hydrogens have a slight positive charge l Molecules with this unequal distribution of charges are called polar molecules l
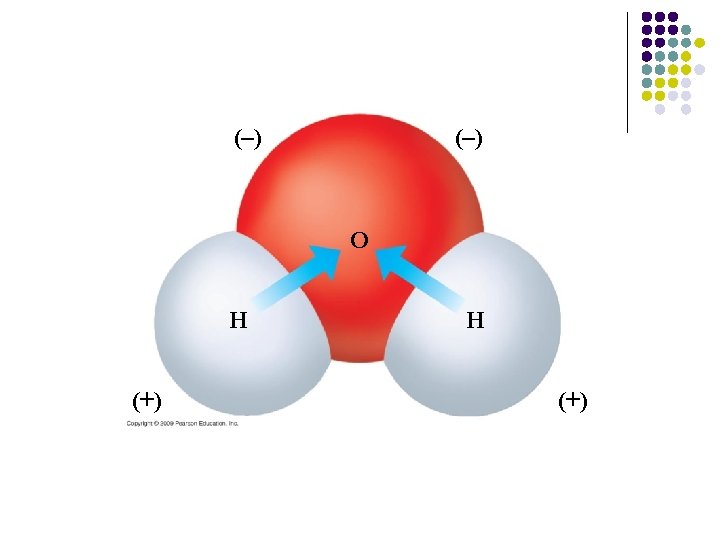

Partial charges result in bonds between molecules l Some chemical bonds are weaker than covalent bonds l Water molecules are electrically attracted to oppositely charged regions on neighboring molecules (may be another H 2 O or a different polar molecule) – Because the positively charged region is always a hydrogen atom, the bond is called a hydrogen bond
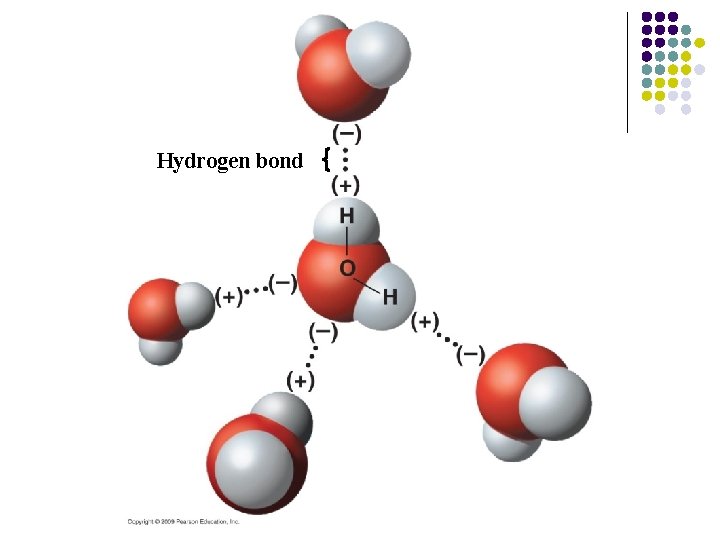
Hydrogen bond
1. Hydrogen bonds make liquid water cohesive l Hydrogen bonding causes molecules to stick together, a property called cohesion – – Cohesion is much stronger for water than other liquids This is useful in plants that depend upon cohesion to help transport water and nutrients up the plant Copyright © 2009 Pearson Education, Inc.
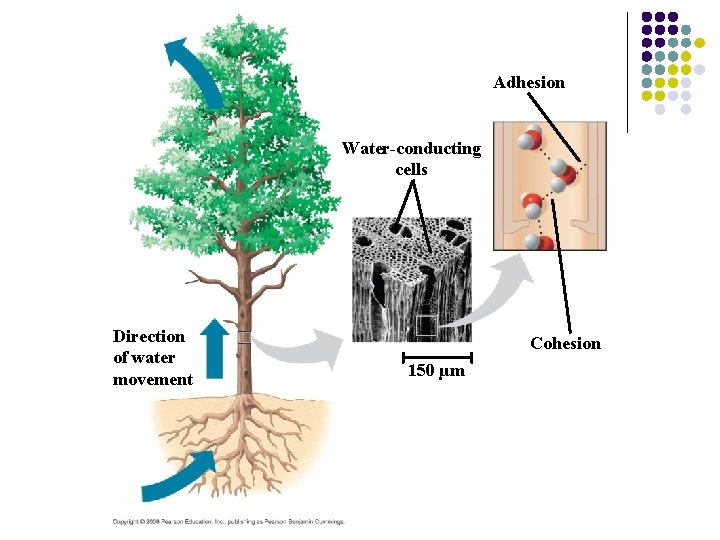
Adhesion Water-conducting cells Direction of water movement Cohesion 150 µm
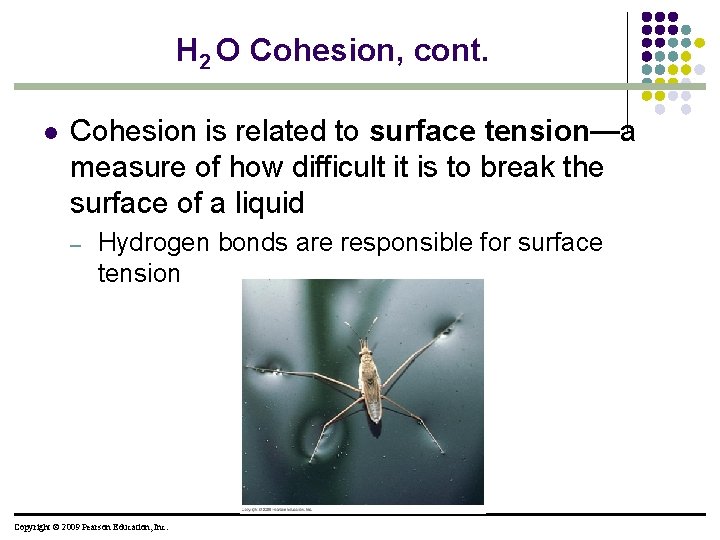
H 2 O Cohesion, cont. l Cohesion is related to surface tension—a measure of how difficult it is to break the surface of a liquid – Hydrogen bonds are responsible for surface tension Copyright © 2009 Pearson Education, Inc.
2. Water’s hydrogen bonds moderate temperature l Because of hydrogen bonding, water has a greater ability to resist temperature change than other liquids – – l Heat is the energy associated with movement of atoms and molecules in matter Temperature measures the intensity of heat Heat must be absorbed to break hydrogen bonds; heat is released when hydrogen bonds form Copyright © 2009 Pearson Education, Inc.

Evaporative cooling! l l Water must absorb a large amount of heat to evaporate. (break the H-bond with other water molecules & turn to gas) As a result, the surface of the liquid that remains behind cools down (hottest molecules gone).

3. Ice is less dense than liquid water l l Water is less dense as a solid due to hydrogen bonding When water freezes, each molecule forms a stable hydrogen bond with four neighbors – – l A three-dimensional crystal results There is space between the water molecules Ice is less dense than water, so it floats and moderates temperatures under the ice. Also provides surface for animals to walk on over the water. Copyright © 2009 Pearson Education, Inc.
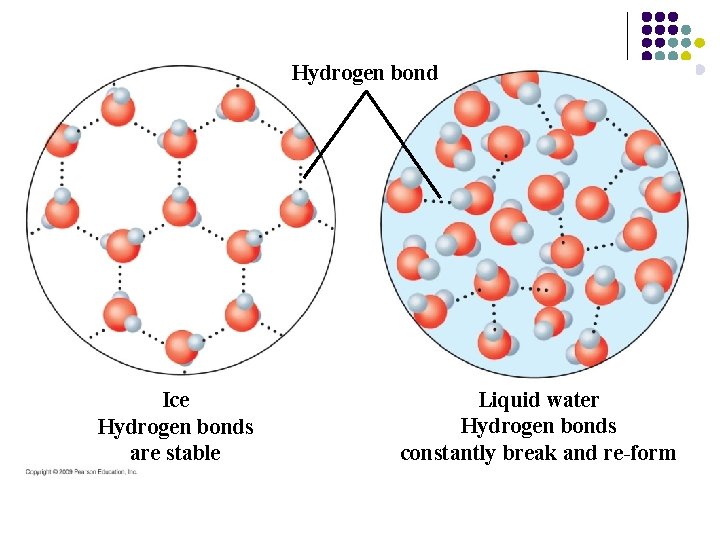
Hydrogen bond Ice Hydrogen bonds are stable Liquid water Hydrogen bonds constantly break and re-form


4. Water is the solvent of life l A solution is a liquid consisting of a uniform mixture of two or more substances – – The dissolving agent is the solvent The substance that is dissolved is the solute Copyright © 2009 Pearson Education, Inc.
4. Water is the solvent of life l Water is a versatile solvent that is fundamental to life processes – – Its versatility results from its polarity Table salt is an example of a solute that will go into solution in water – Sodium and chloride ions and water are attracted to each other because of their charges Copyright © 2009 Pearson Education, Inc.
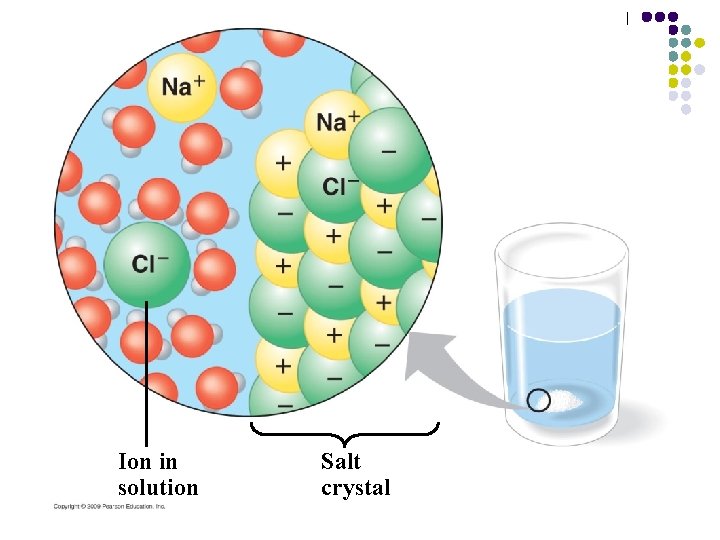
Ion in solution Salt crystal
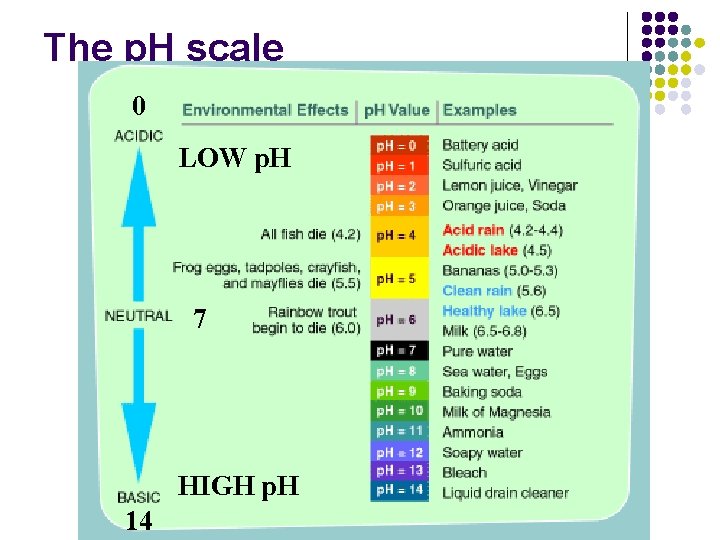
The p. H scale 0 LOW p. H 7 HIGH p. H 14
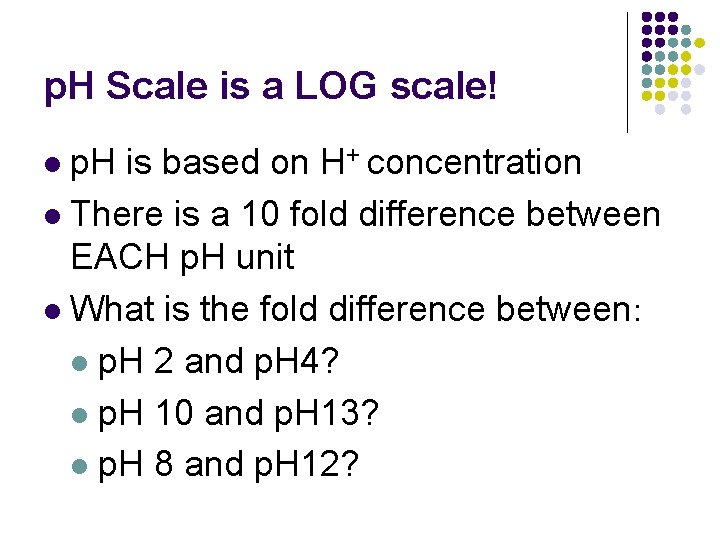
p. H Scale is a LOG scale! p. H is based on H+ concentration l There is a 10 fold difference between EACH p. H unit l What is the fold difference between: l p. H 2 and p. H 4? l p. H 10 and p. H 13? l p. H 8 and p. H 12? l
- Slides: 37