Biochemistry Functional Groups and Macromolecules CarbonThe Backbone of
Biochemistry Functional Groups and Macromolecules
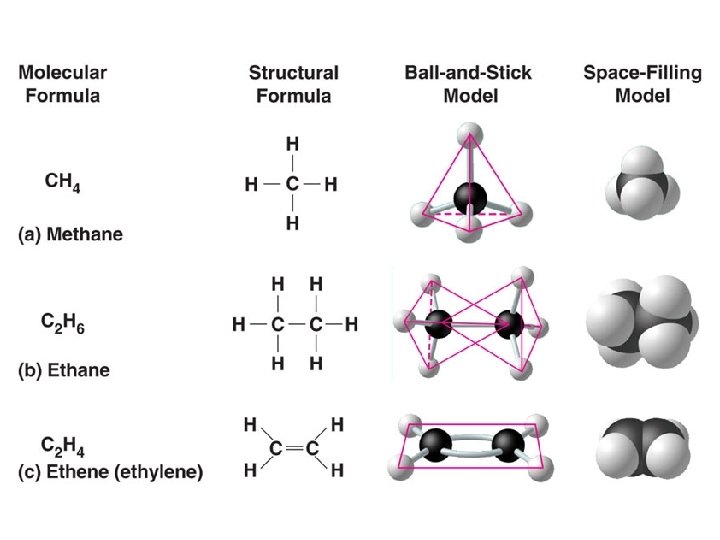
Carbon—The Backbone of Biological Molecules n All living organisms n. Are made up of chemicals based mostly on carbon due to its bonding ability n. All life considered “carbon based lifeforms”
Biochemistry Part 1 Functional Groups

Vocab to know… • Organic chemistry – the study of carbon compounds • Organic compounds have carbon in them (& usually H) – Exception: CO 2 is considered INORGANIC –Range from simple to big molecules
Formation of Bonds w/ Carbon • Carbon atoms – forms diverse molecules –b/c carbon has 4 valence electrons bind to to 4 other atoms – can form 4 covalent bonds with itself or other atoms very important in living things! • Carbon has bonding versatility – allows it to form many diverse molecules, including carbon skeletons (carbon “chains”)
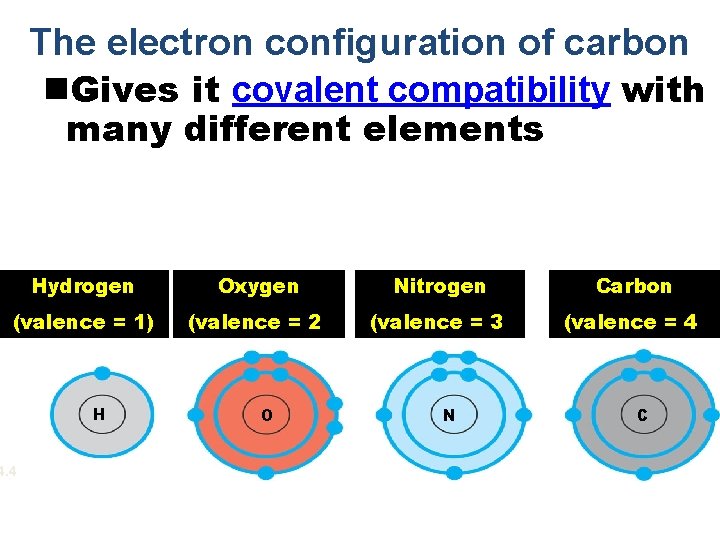
The electron configuration of carbon n. Gives it covalent compatibility with many different elements Hydrogen Oxygen Nitrogen Carbon (valence = 1) (valence = 2) (valence = 3) (valence = 4) 4. 4 H O N C
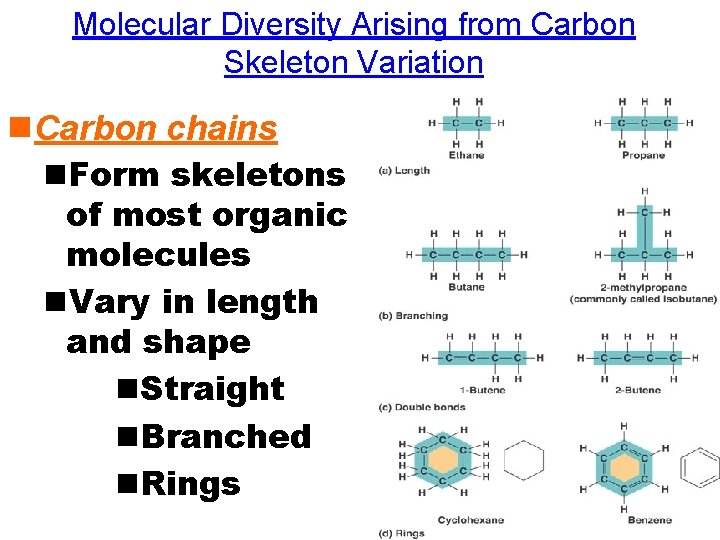
Molecular Diversity Arising from Carbon Skeleton Variation n. Carbon chains n. Form skeletons of most organic molecules n. Vary in length and shape n. Straight n. Branched n. Rings
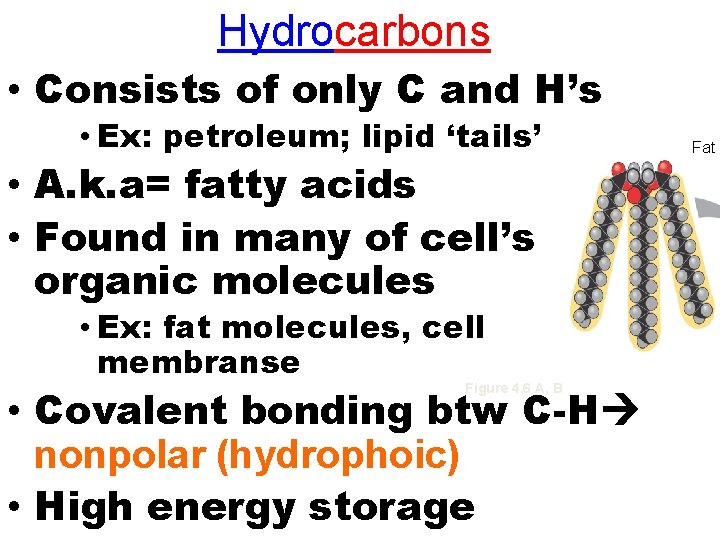
Hydrocarbons • Consists of only C and H’s • Ex: petroleum; lipid ‘tails’ • A. k. a= fatty acids • Found in many of cell’s organic molecules • Ex: fat molecules, cell membranse • Covalent bonding btw C-H nonpolar (hydrophoic) • High energy storage Figure 4. 6 A, B Fat d
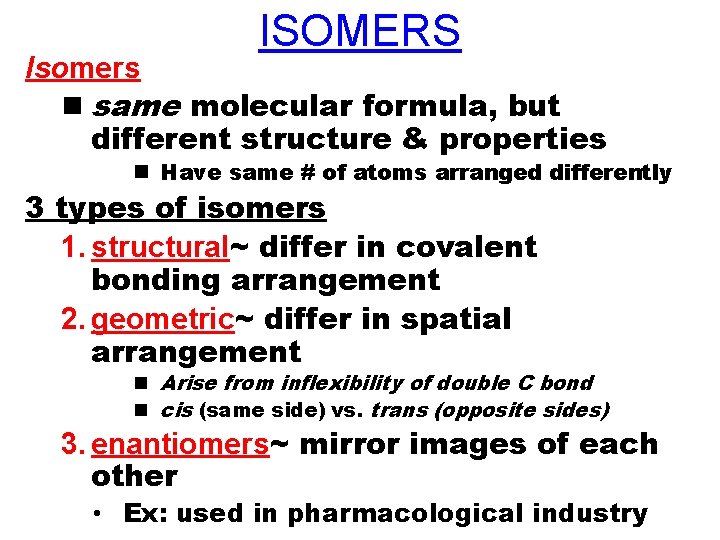
ISOMERS Isomers n same molecular formula, but different structure & properties n Have same # of atoms arranged differently 3 types of isomers 1. structural~ differ in covalent bonding arrangement 2. geometric~ differ in spatial arrangement n Arise from inflexibility of double C bond n cis (same side) vs. trans (opposite sides) 3. enantiomers~ mirror images of each other • Ex: used in pharmacological industry
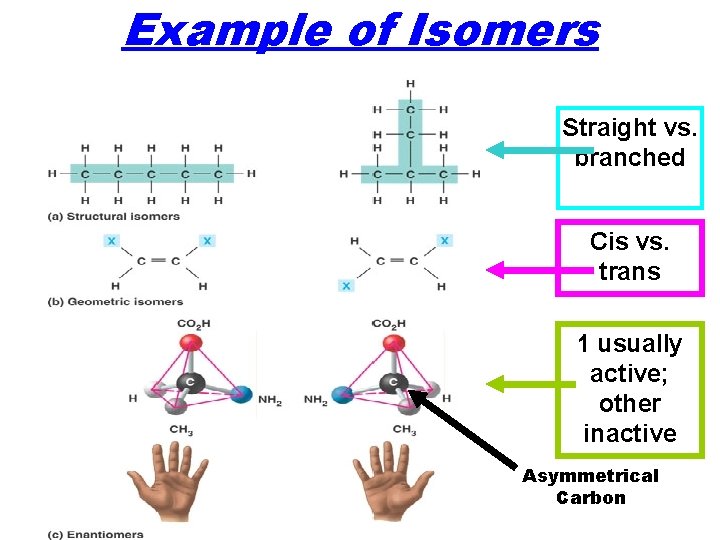
Example of Isomers Straight vs. branched Cis vs. trans 1 usually active; other inactive Asymmetrical Carbon
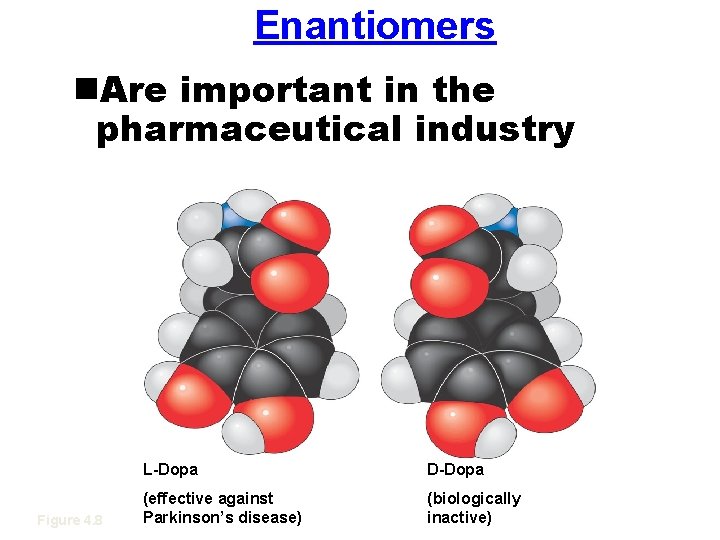
Enantiomers n. Are important in the pharmaceutical industry Figure 4. 8 L-Dopa D-Dopa (effective against Parkinson’s disease) (biologically inactive)
Functional Groups – 7 different groups in biology – part of organic molecules – involved in chemical rxns • chemically reactive groups – Each group behaves in a consistent fashion no matter where it is – # & arrangement of groups helps give molecules unique, distinctive chemical properties
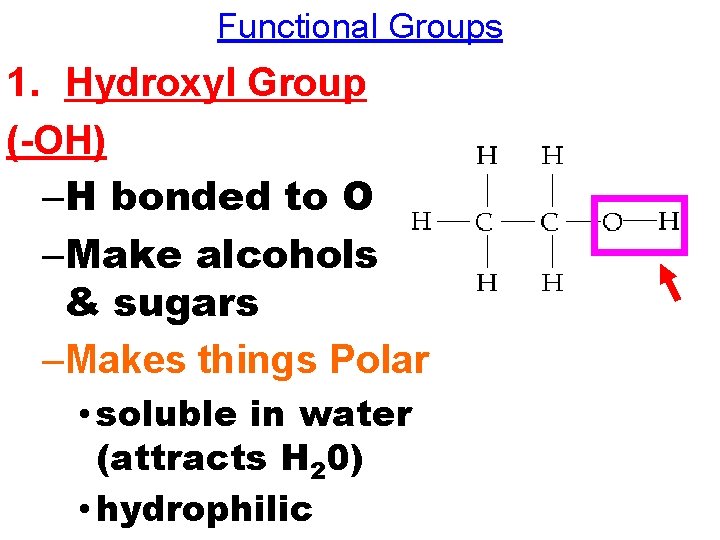
Functional Groups 1. Hydroxyl Group (-OH) –H bonded to O –Make alcohols & sugars –Makes things Polar • soluble in water (attracts H 20) • hydrophilic
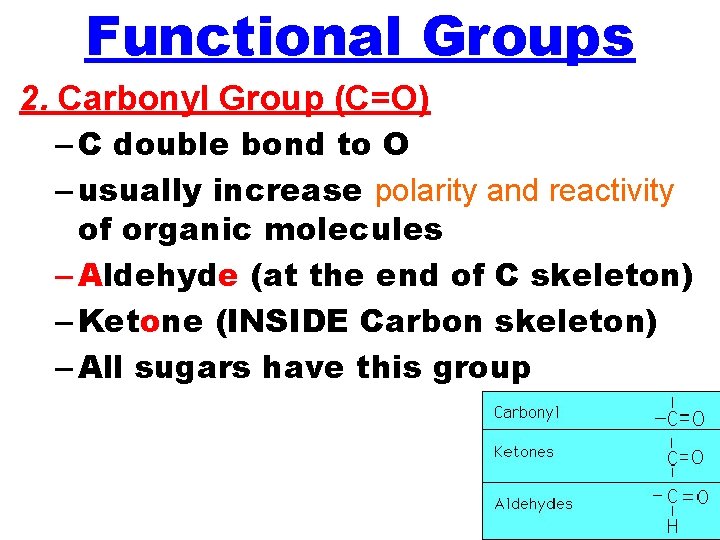
Functional Groups 2. Carbonyl Group (C=O) – C double bond to O – usually increase polarity and reactivity of organic molecules – Aldehyde (at the end of C skeleton) – Ketone (INSIDE Carbon skeleton) – All sugars have this group
• Aldehyde • Ketone – **Think: all the way at the end!
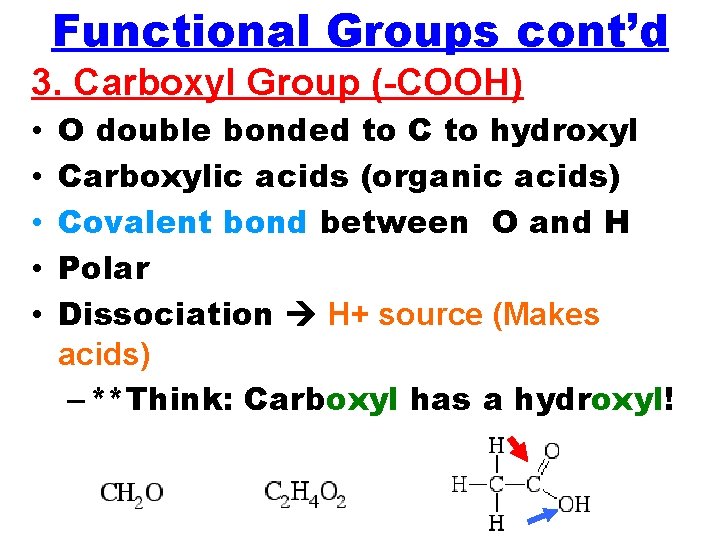
Functional Groups cont’d 3. Carboxyl Group (-COOH) • • • O double bonded to C to hydroxyl Carboxylic acids (organic acids) Covalent bond between O and H Polar Dissociation H+ source (Makes acids) – **Think: Carboxyl has a hydroxyl!
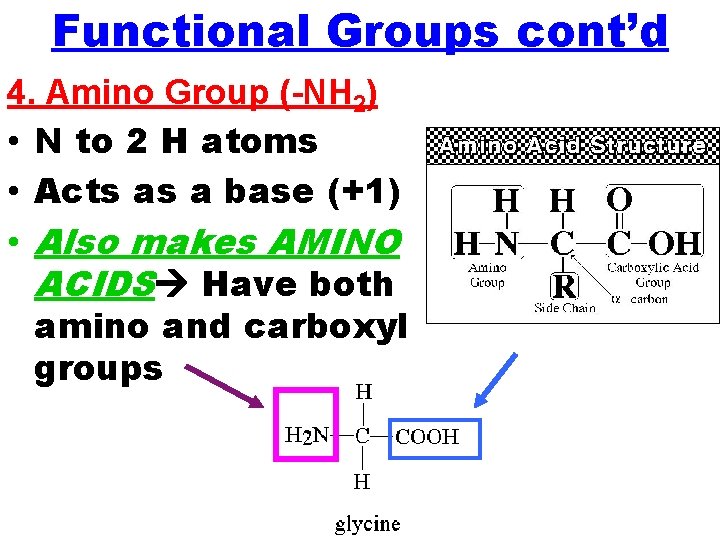
Functional Groups cont’d 4. Amino Group (-NH 2) • N to 2 H atoms • Acts as a base (+1) • Also makes AMINO ACIDS Have both amino and carboxyl groups
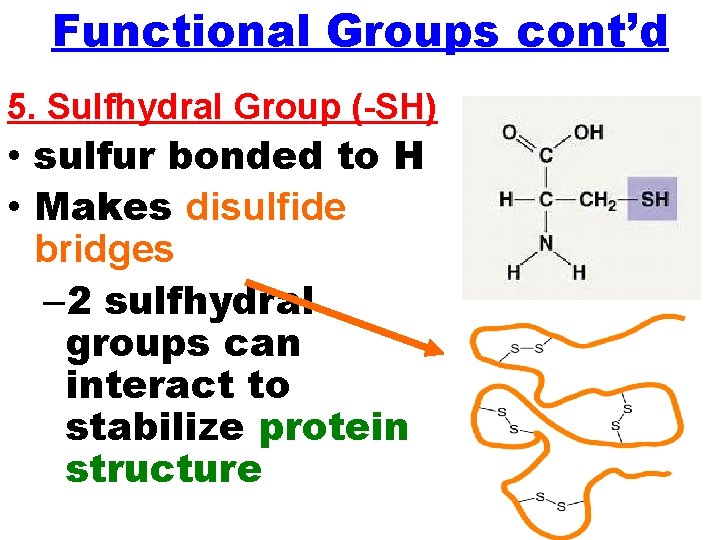
Functional Groups cont’d 5. Sulfhydral Group (-SH) • sulfur bonded to H • Makes disulfide bridges – 2 sulfhydral groups can interact to stabilize protein structure
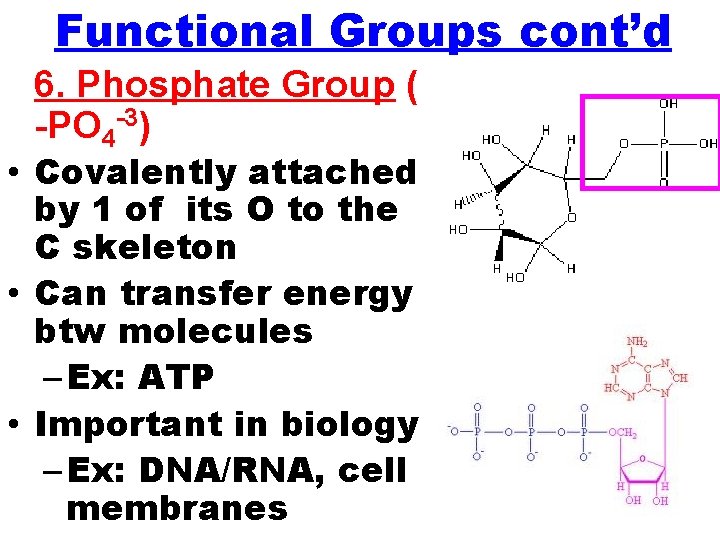
Functional Groups cont’d 6. Phosphate Group ( -PO 4 -3) • Covalently attached by 1 of its O to the C skeleton • Can transfer energy btw molecules – Ex: ATP • Important in biology – Ex: DNA/RNA, cell membranes
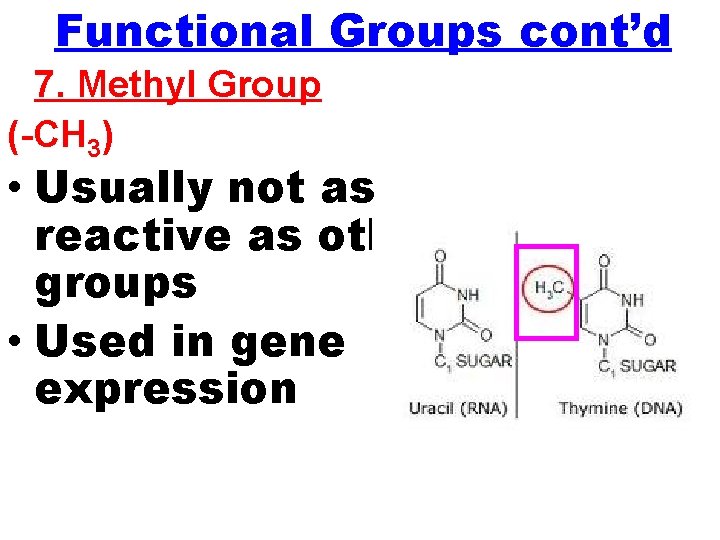
Functional Groups cont’d 7. Methyl Group (-CH 3) • Usually not as reactive as other groups • Used in gene expression
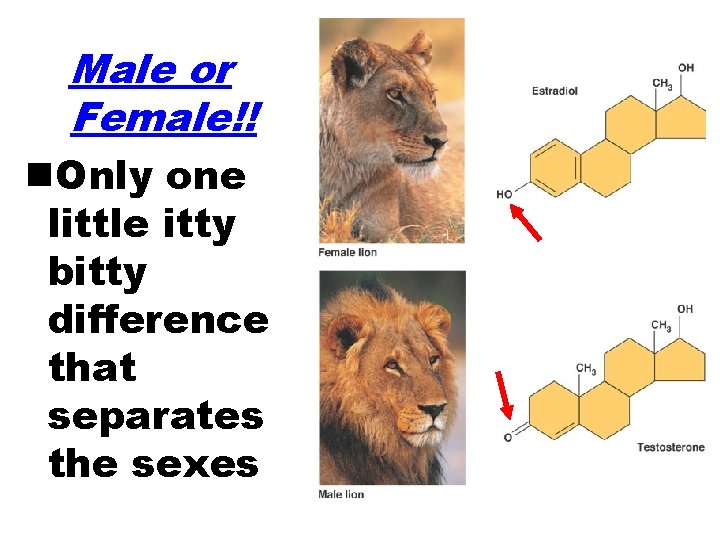
Male or Female!! n. Only one little itty bitty difference that separates the sexes
- Slides: 22