Biochemistry Functional Groups and Macromolecules Biochemistry Part 2
Biochemistry Functional Groups and Macromolecules
Biochemistry Part 2: Macromolecules
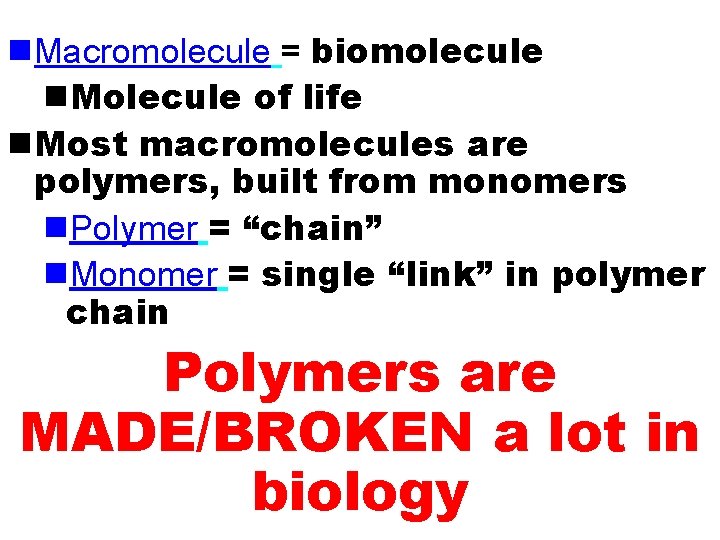
n. Macromolecule = biomolecule n. Molecule of life n. Most macromolecules are polymers, built from monomers n. Polymer = “chain” n. Monomer = single “link” in polymer chain Polymers are MADE/BROKEN a lot in biology
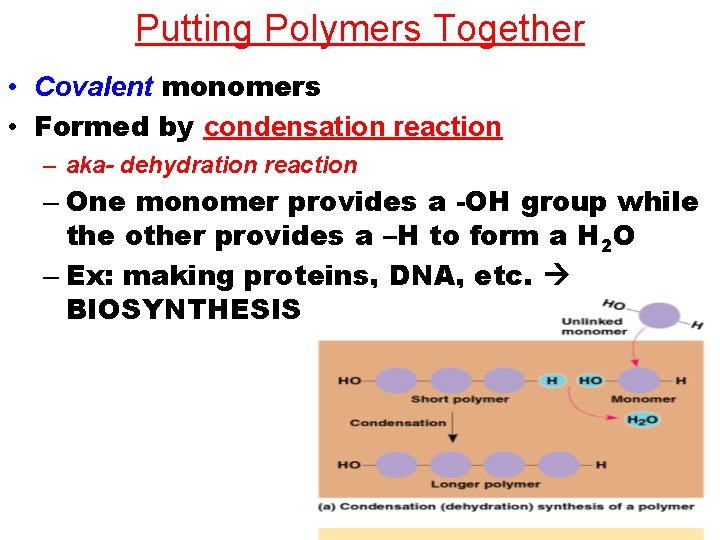
Putting Polymers Together • Covalent monomers • Formed by condensation reaction – aka- dehydration reaction – One monomer provides a -OH group while the other provides a –H to form a H 2 O – Ex: making proteins, DNA, etc. BIOSYNTHESIS
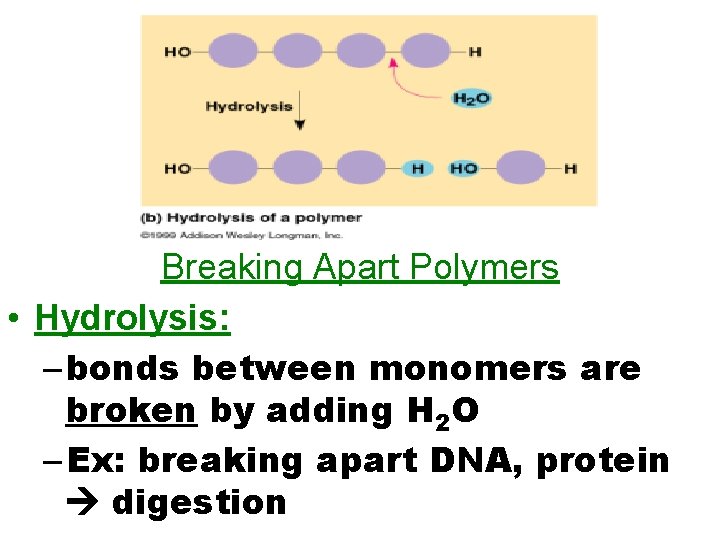
Breaking Apart Polymers • Hydrolysis: – bonds between monomers are broken by adding H 2 O – Ex: breaking apart DNA, protein digestion
There are 4 macromolecules needed for life
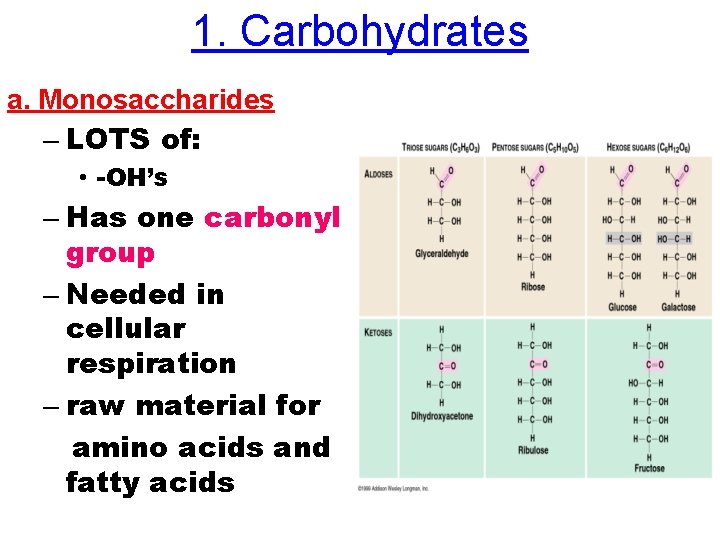
1. Carbohydrates a. Monosaccharides – LOTS of: • -OH’s – Has one carbonyl group – Needed in cellular respiration – raw material for amino acids and fatty acids
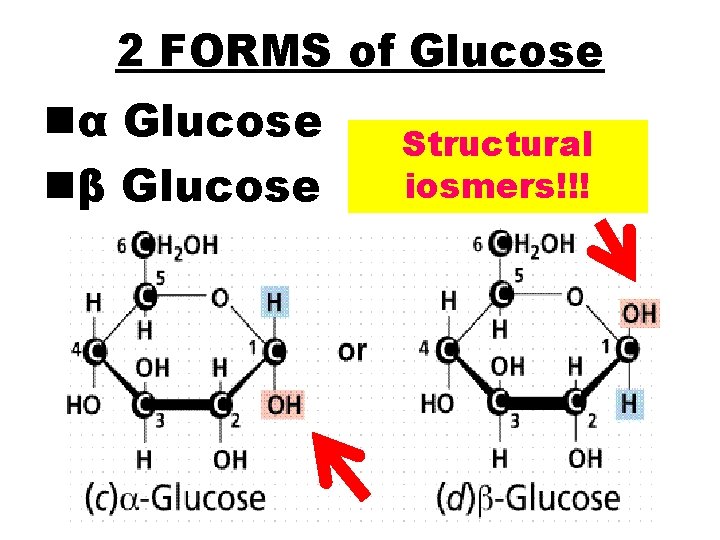
2 FORMS of Glucose nα Glucose nβ Glucose Structural iosmers!!!
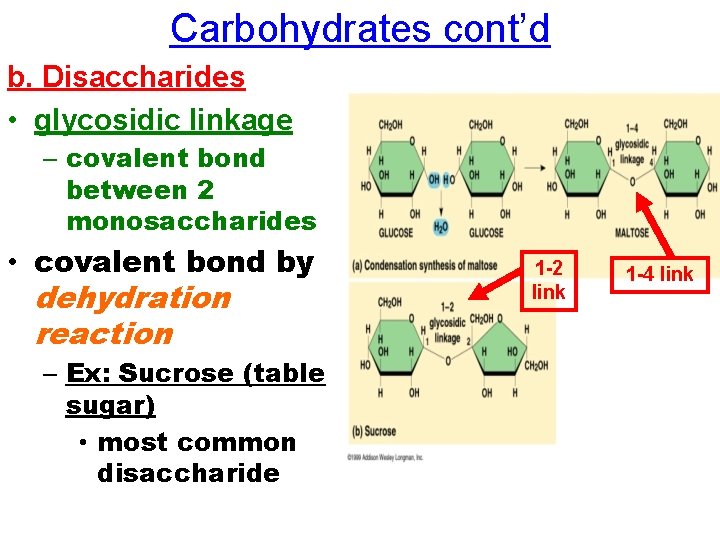
Carbohydrates cont’d b. Disaccharides • glycosidic linkage – covalent bond between 2 monosaccharides • covalent bond by dehydration reaction – Ex: Sucrose (table sugar) • most common disaccharide 1 -2 link 1 -4 link
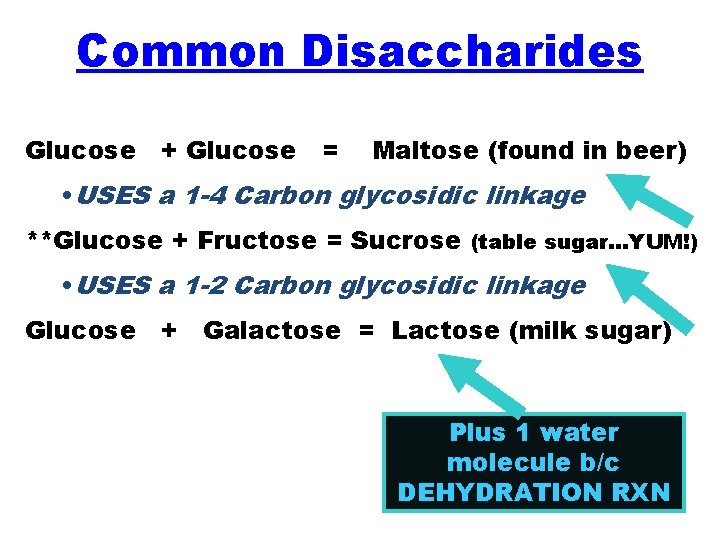
Common Disaccharides Glucose + Glucose = Maltose (found in beer) • USES a 1 -4 Carbon glycosidic linkage **Glucose + Fructose = Sucrose (table sugar…YUM!) • USES a 1 -2 Carbon glycosidic linkage Glucose + Galactose = Lactose (milk sugar) Plus 1 water molecule b/c DEHYDRATION RXN
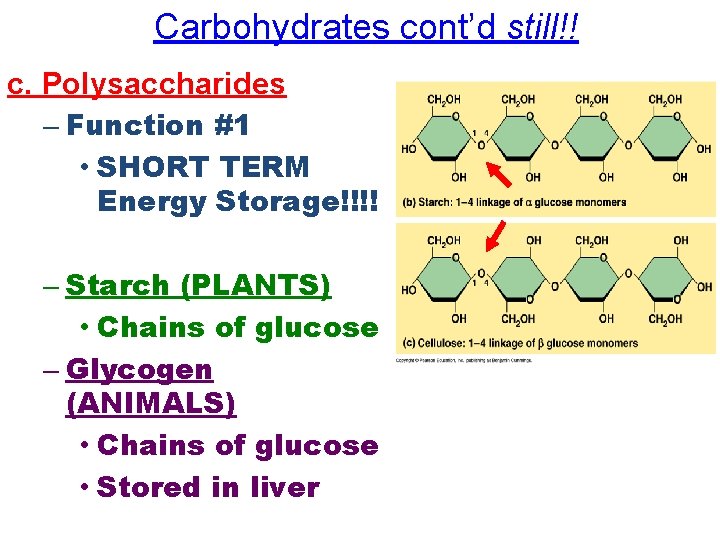
Carbohydrates cont’d still!! c. Polysaccharides – Function #1 • SHORT TERM Energy Storage!!!! – Starch (PLANTS) • Chains of glucose – Glycogen (ANIMALS) • Chains of glucose • Stored in liver

Carbohydrates cont’d still!! c. Polysaccharides (con’t) – Function #2 • Structural/Support!!!! – Cellulose (aka –Fiber… to humans) • most abundant compound in world • Wood and cell walls – Chitin • Insect exoskeletons • Cell walls of fungi • Surgical thread

2. Lipids • Not polymers • • – Instead glycerol + fatty acid Includes fats, phospholipids, steroids Hydrophobic (NON-POLAR) Non-polar C-H bonds in fatty acid ‘tails’ Ester linkage: covalent bonds in lipids
Function of Lipids • Main Function – Lipids= long term energy storage like carbs but carbs = short-term usage Other functions: • Insulation • Protection (membranes) • Chemical signals
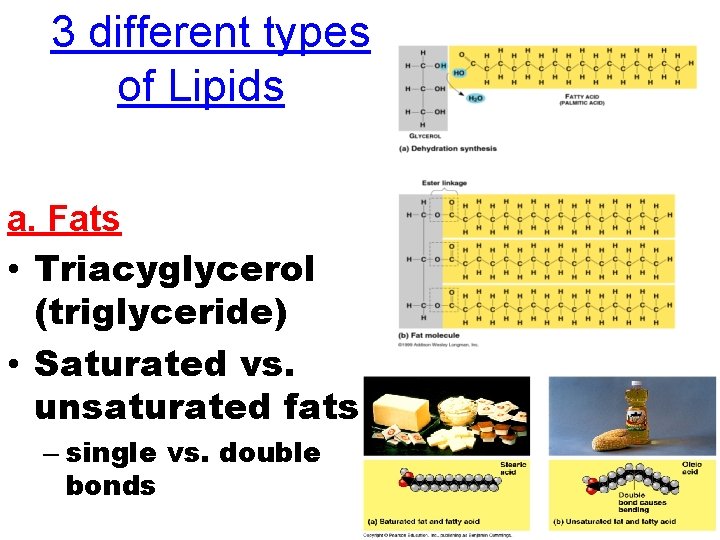
3 different types of Lipids a. Fats • Triacyglycerol (triglyceride) • Saturated vs. unsaturated fats – single vs. double bonds
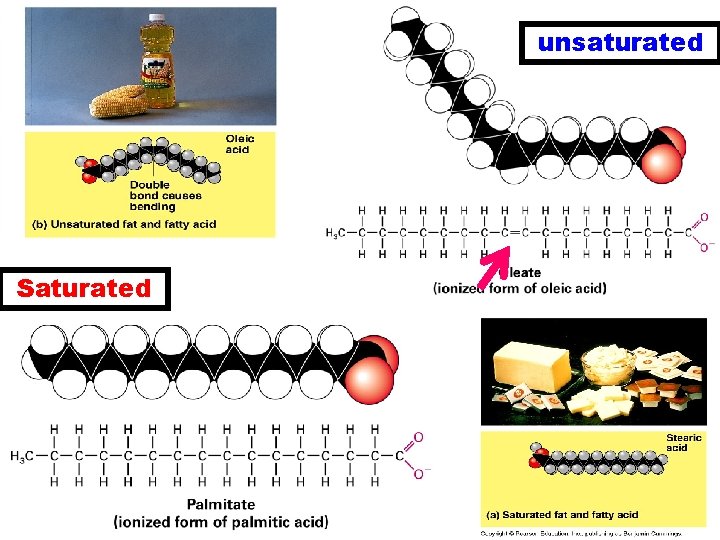
unsaturated Saturated
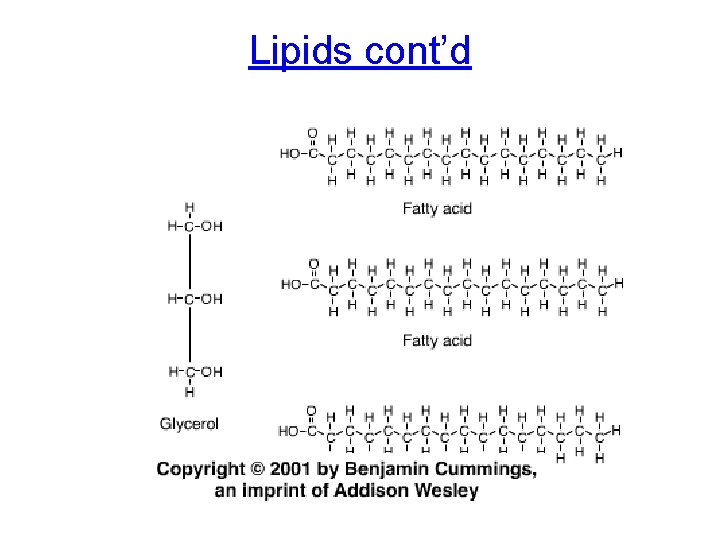
Lipids cont’d
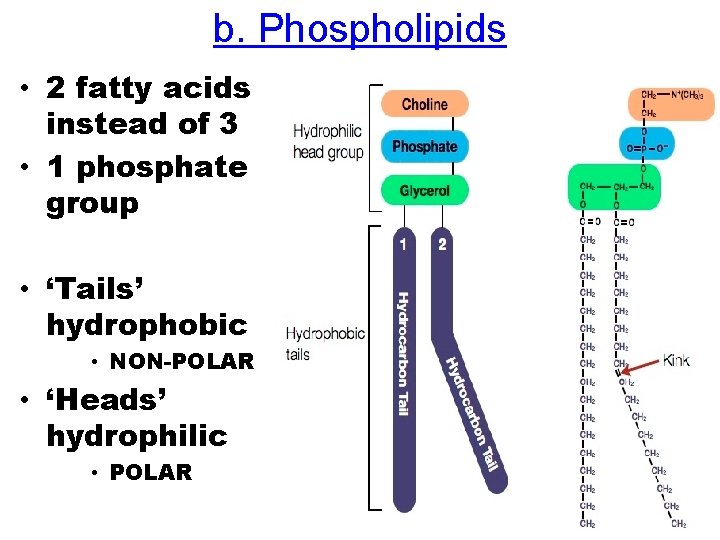
b. Phospholipids • 2 fatty acids instead of 3 • 1 phosphate group • ‘Tails’ hydrophobic • NON-POLAR • ‘Heads’ hydrophilic • POLAR
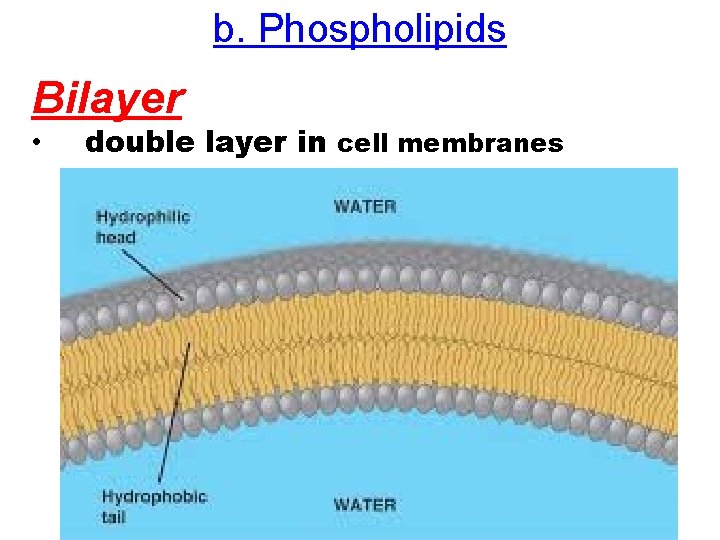
b. Phospholipids Bilayer • double layer in cell membranes
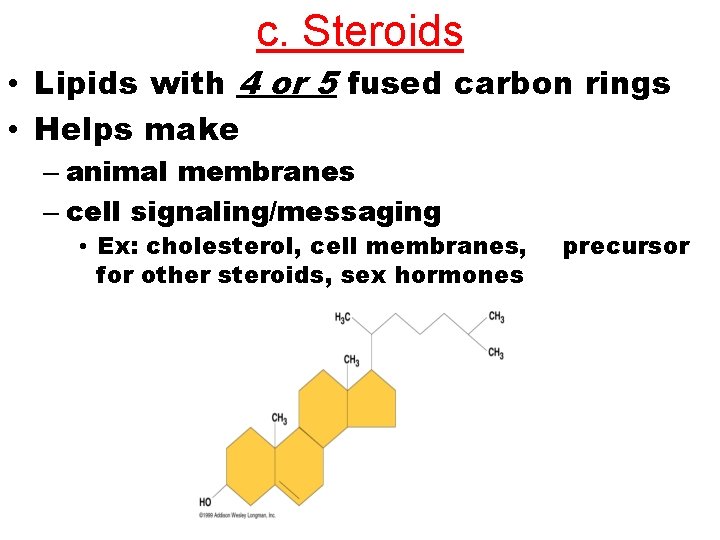
c. Steroids • Lipids with 4 or 5 fused carbon rings • Helps make – animal membranes – cell signaling/messaging • Ex: cholesterol, cell membranes, for other steroids, sex hormones precursor
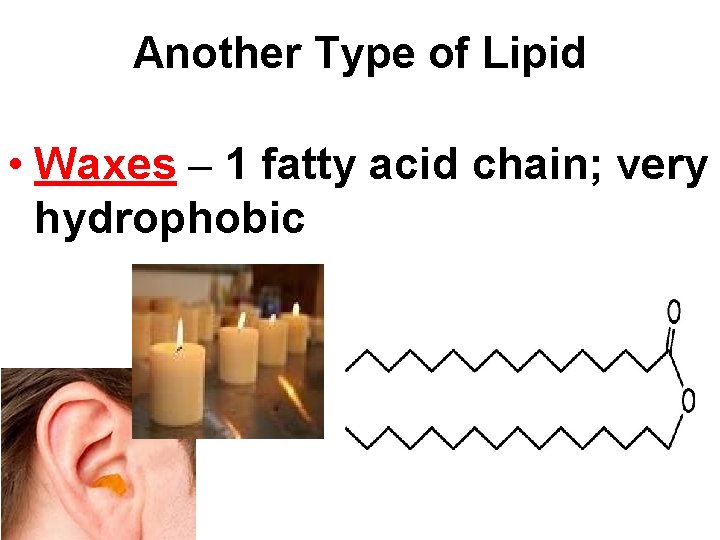
Another Type of Lipid • Waxes – 1 fatty acid chain; very hydrophobic
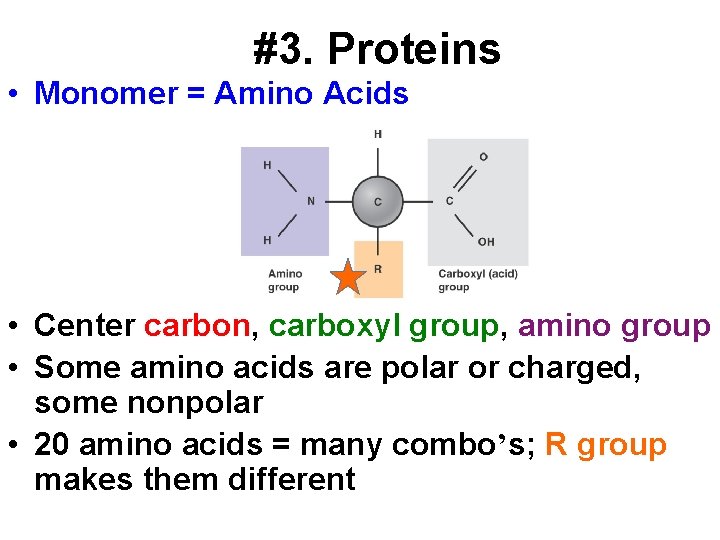
#3. Proteins • Monomer = Amino Acids • Center carbon, carboxyl group, amino group • Some amino acids are polar or charged, some nonpolar • 20 amino acids = many combo’s; R group makes them different
Proteins (con’t) R group characteristics • Some are: – polar (hydrophilic) – nonpolar (hydrophobic) – Acidic – Basic • ALL proteins have a 3 D shape (called its conformation) • Form polypeptides – peptide bonds (covalent bond) are created
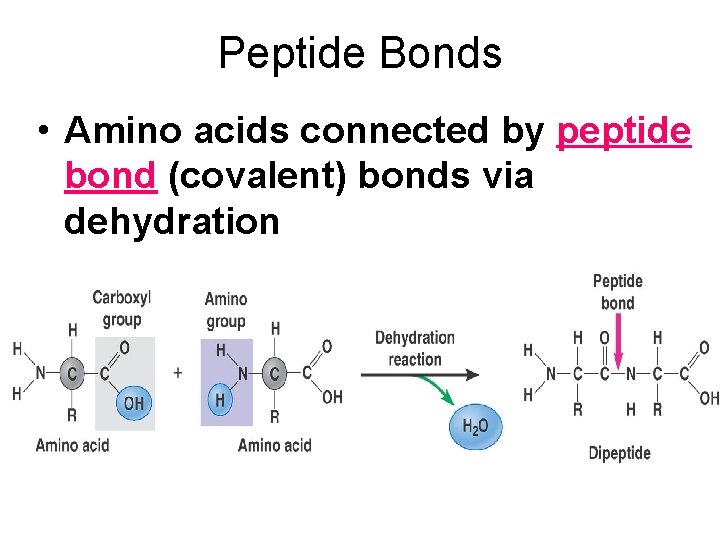
Peptide Bonds • Amino acids connected by peptide bond (covalent) bonds via dehydration

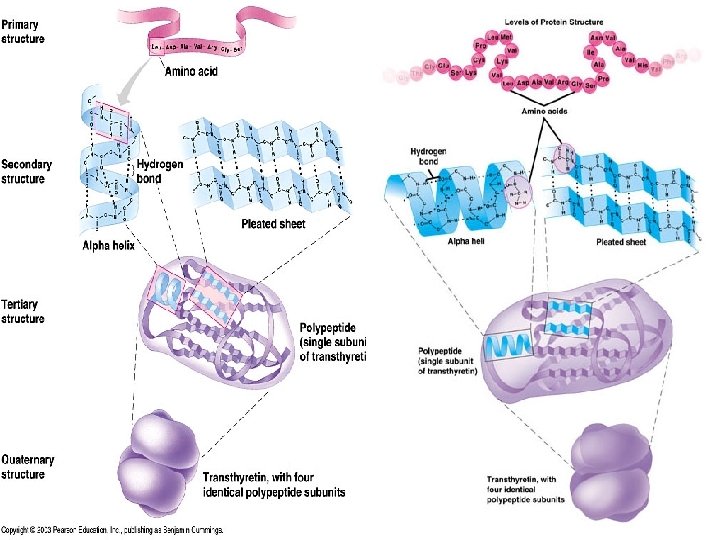
Proteins (con’t) Proteins have 3 (and sometimes 4) for LEVELS of conformation
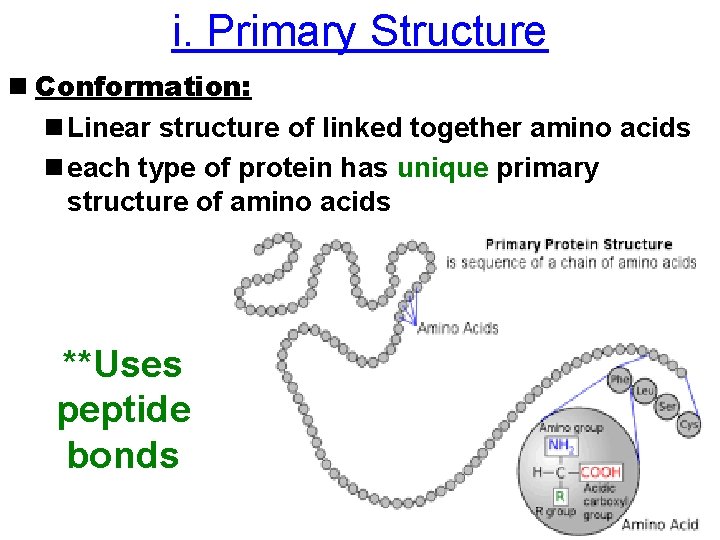
i. Primary Structure n Conformation: n Linear structure of linked together amino acids n each type of protein has unique primary structure of amino acids **Uses peptide bonds
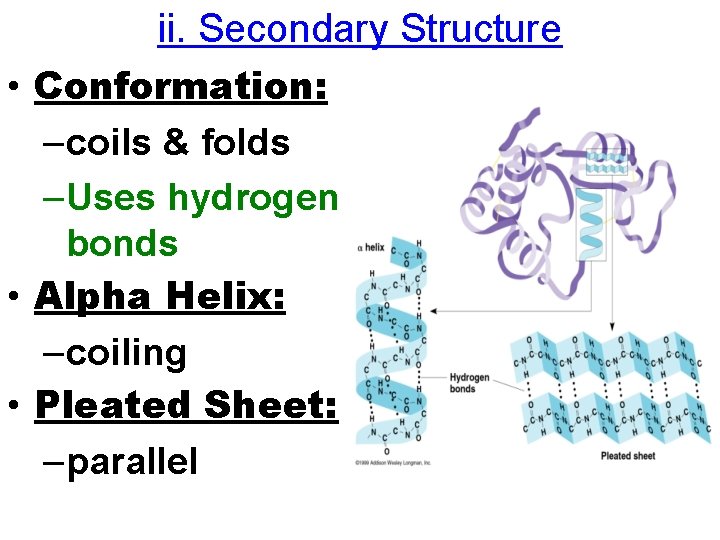
ii. Secondary Structure • Conformation: –coils & folds –Uses hydrogen bonds • Alpha Helix: –coiling • Pleated Sheet: –parallel
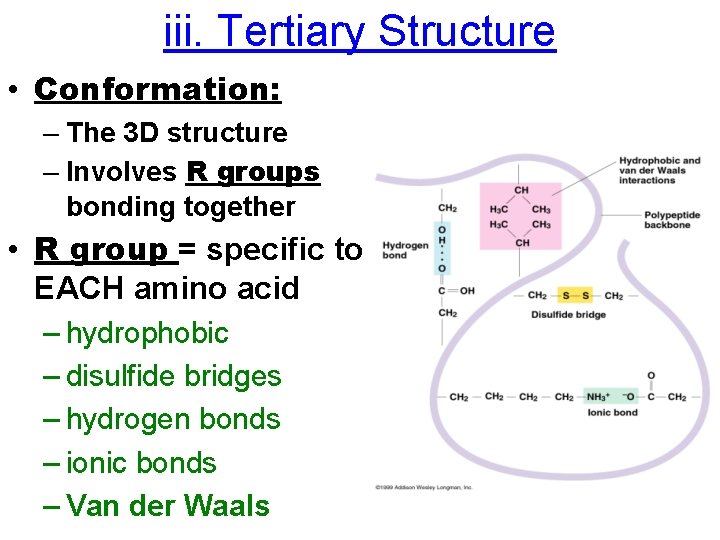
iii. Tertiary Structure • Conformation: – The 3 D structure – Involves R groups bonding together • R group = specific to EACH amino acid – hydrophobic – disulfide bridges – hydrogen bonds – ionic bonds – Van der Waals
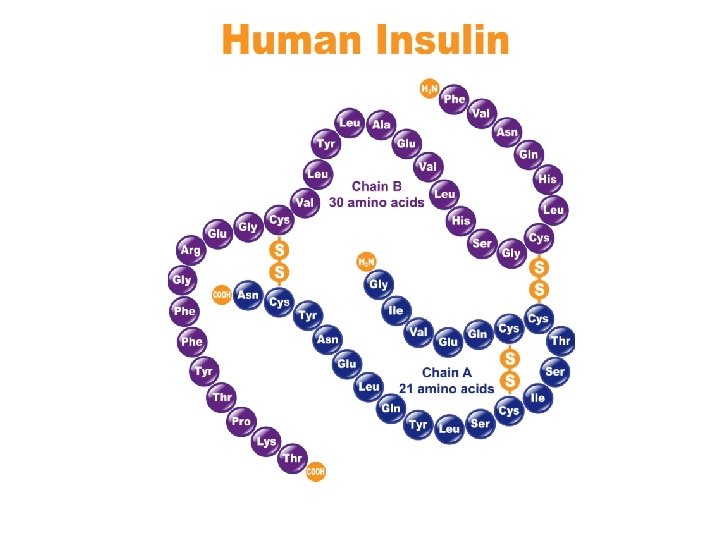
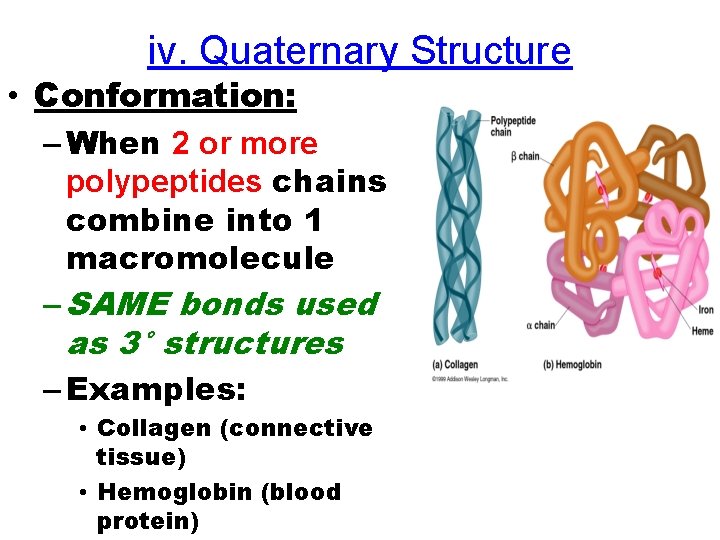
iv. Quaternary Structure • Conformation: – When 2 or more polypeptides chains combine into 1 macromolecule – SAME bonds used as 3° structures – Examples: • Collagen (connective tissue) • Hemoglobin (blood protein)
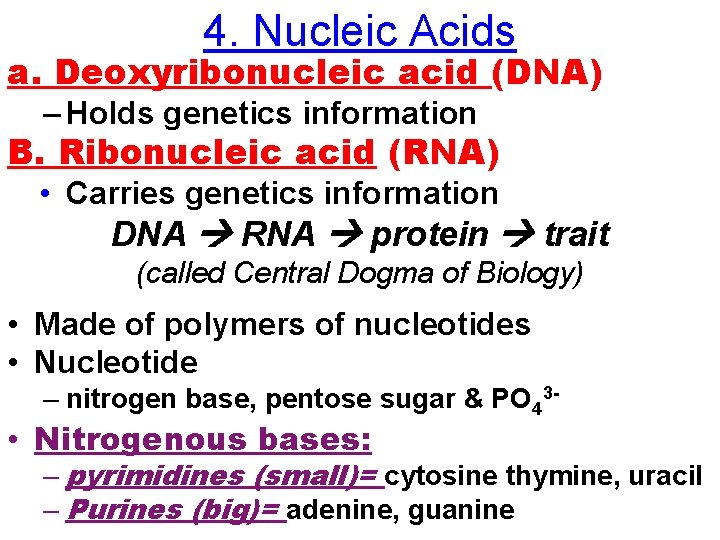
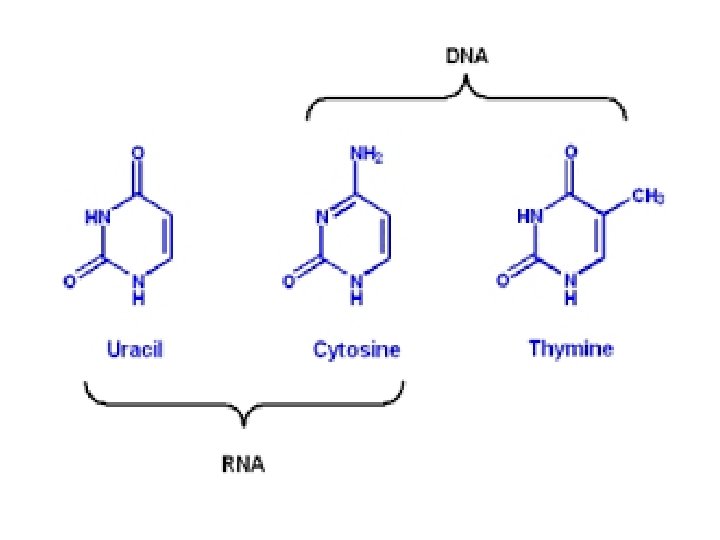
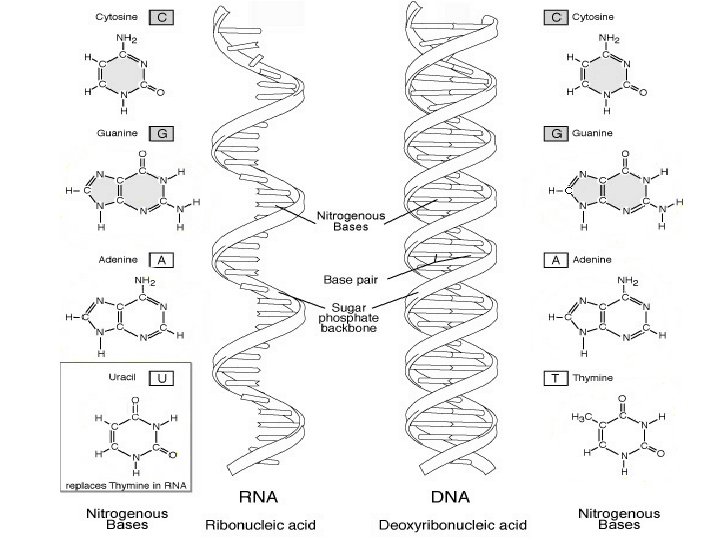
4. Nucleic Acids a. Deoxyribonucleic acid (DNA) – Holds genetics information B. Ribonucleic acid (RNA) • Carries genetics information DNA RNA protein trait (called Central Dogma of Biology) • Made of polymers of nucleotides • Nucleotide – nitrogen base, pentose sugar & PO 43 - • Nitrogenous bases: – pyrimidines (small)= cytosine thymine, uracil – Purines (big)= adenine, guanine
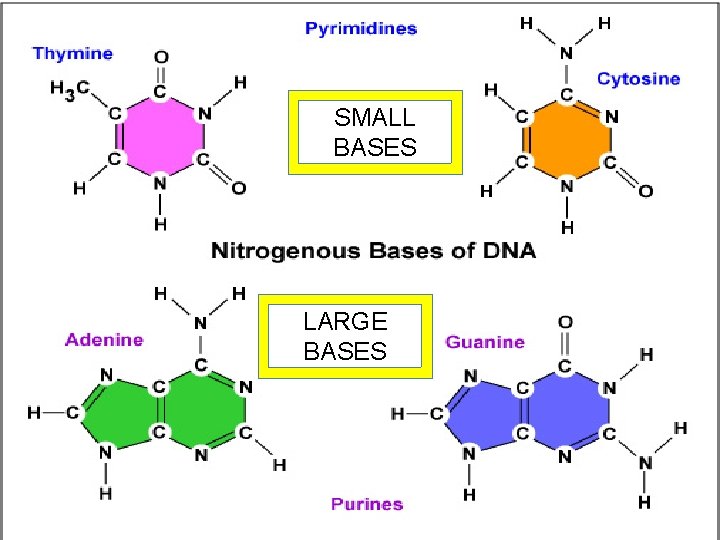
SMALL BASES LARGE BASES
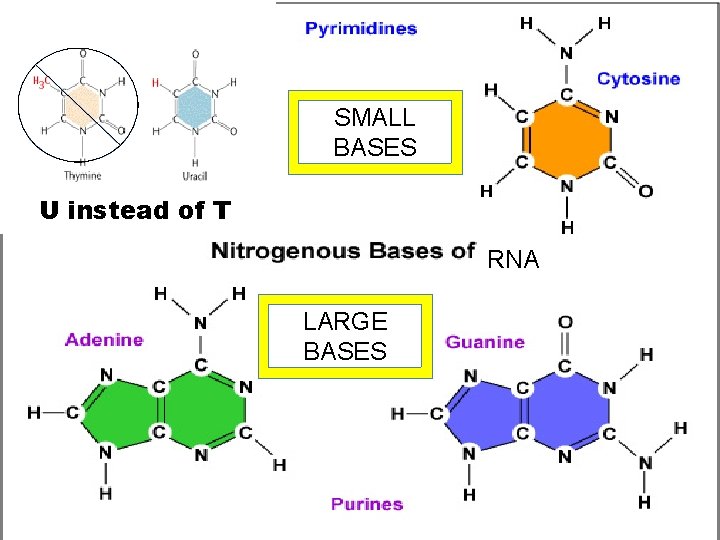
SMALL BASES U instead of T RNA LARGE BASES
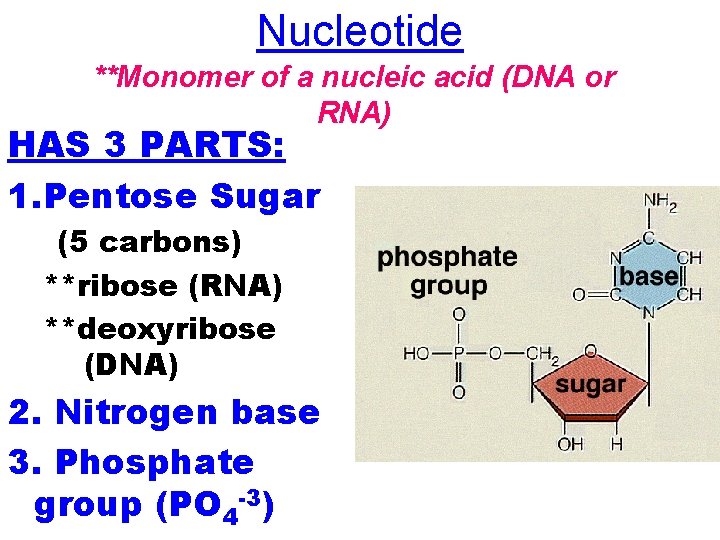
Nucleotide **Monomer of a nucleic acid (DNA or RNA) HAS 3 PARTS: 1. Pentose Sugar (5 carbons) **ribose (RNA) **deoxyribose (DNA) 2. Nitrogen base 3. Phosphate group (PO 4 -3)
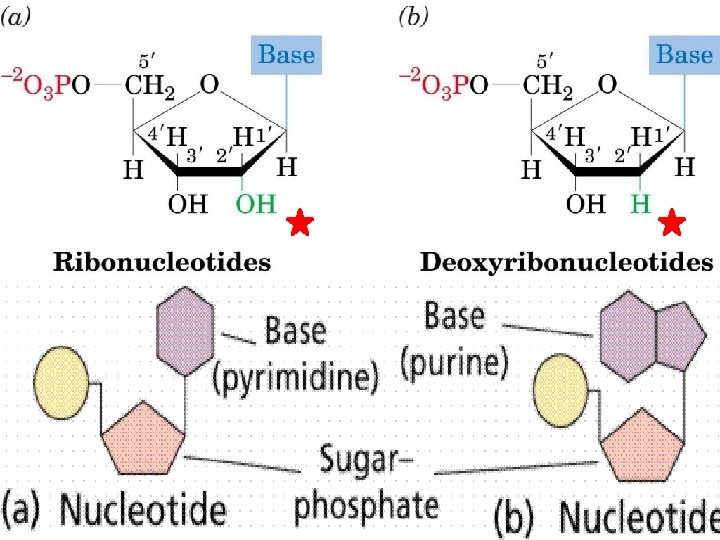
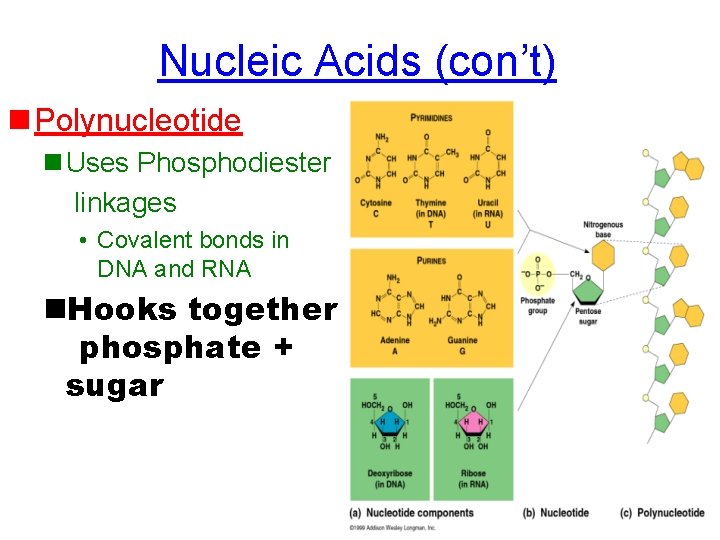
Nucleic Acids (con’t) n Polynucleotide n Uses Phosphodiester linkages • Covalent bonds in DNA and RNA n. Hooks together phosphate + sugar
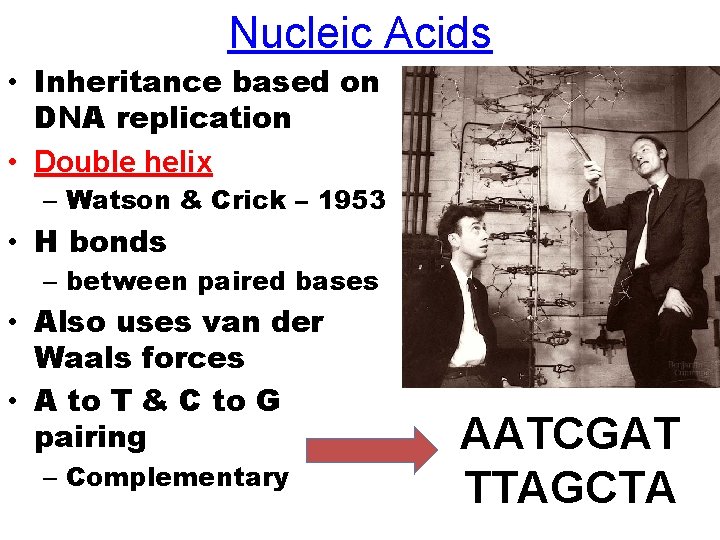
Nucleic Acids • Inheritance based on DNA replication • Double helix – Watson & Crick – 1953 • H bonds – between paired bases • Also uses van der Waals forces • A to T & C to G pairing – Complementary AATCGAT TTAGCTA
- Slides: 41