Biochemistry Essentials for Life n Organic vs Inorganic
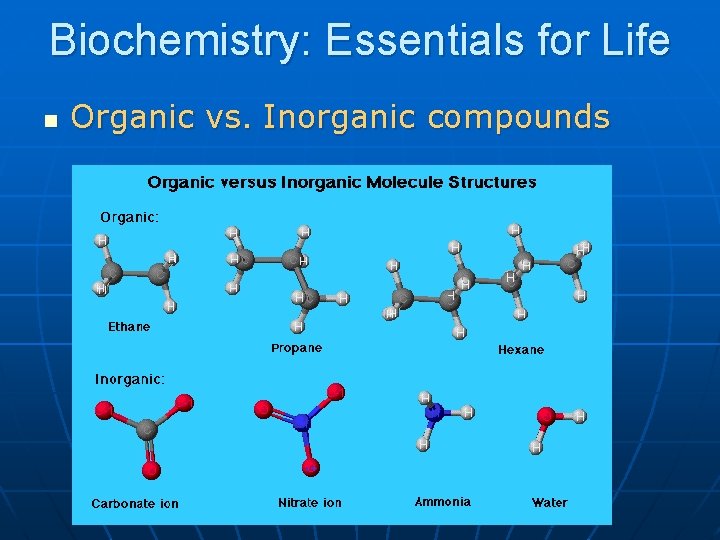
Biochemistry: Essentials for Life n Organic vs. Inorganic compounds
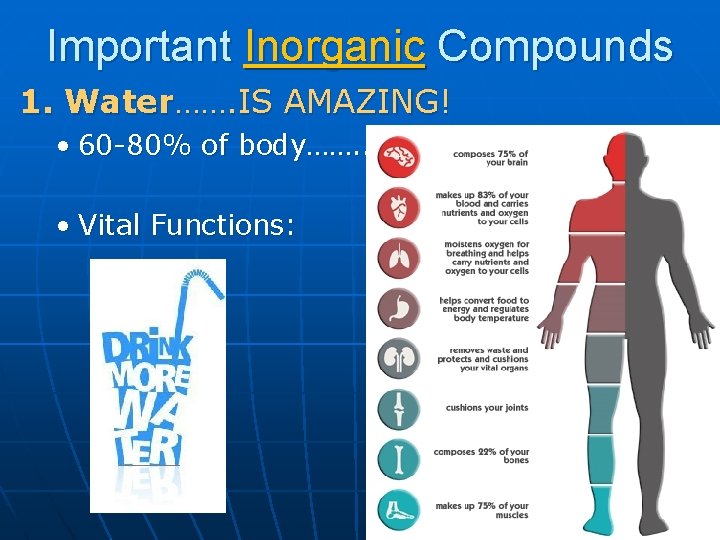
Important Inorganic Compounds 1. Water……. IS AMAZING! • 60 -80% of body……. . Coincidence? • Vital Functions:

Journal n Based on lecture and AMAZING demos WHY is water found in such high amounts in the body? • Remember: structure determines function
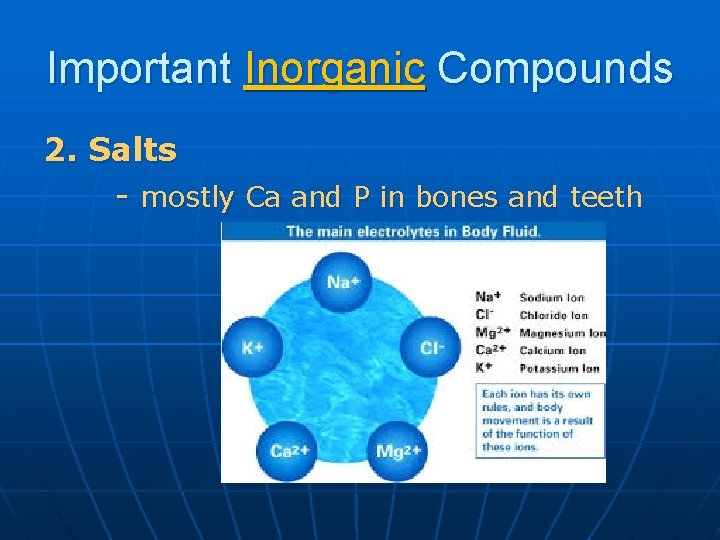
Important Inorganic Compounds 2. Salts - mostly Ca and P in bones and teeth

Important Inorganic Compounds 3. Acids and Bases n Neutralization Reaction • HCl + Na. OH Na. Cl + H 2 O • Homeostasis?
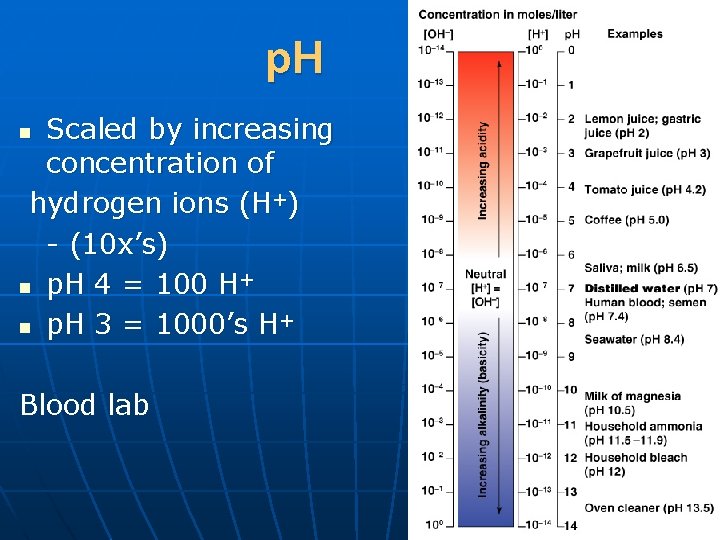
p. H Scaled by increasing concentration of hydrogen ions (H+) - (10 x’s) n p. H 4 = 100 H+ n p. H 3 = 1000’s H+ n Blood lab Figure 2. 11
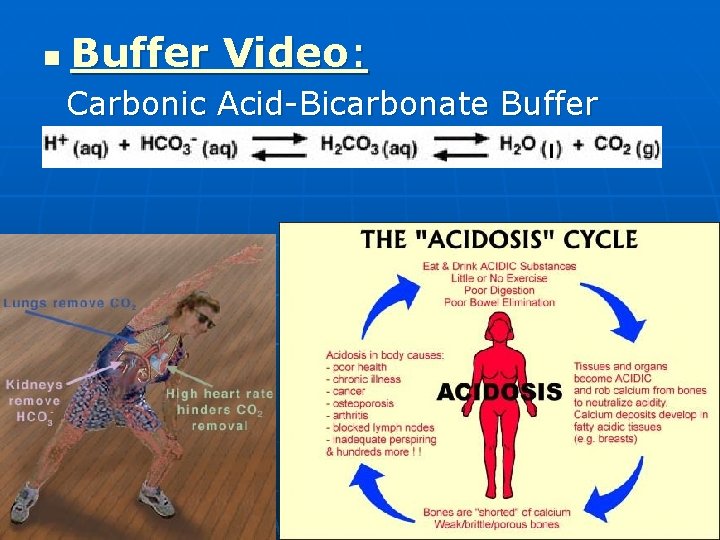
n Buffer Video: Carbonic Acid-Bicarbonate Buffer

Quick Write n Give examples of inorganic substances and the role they have in the human body.
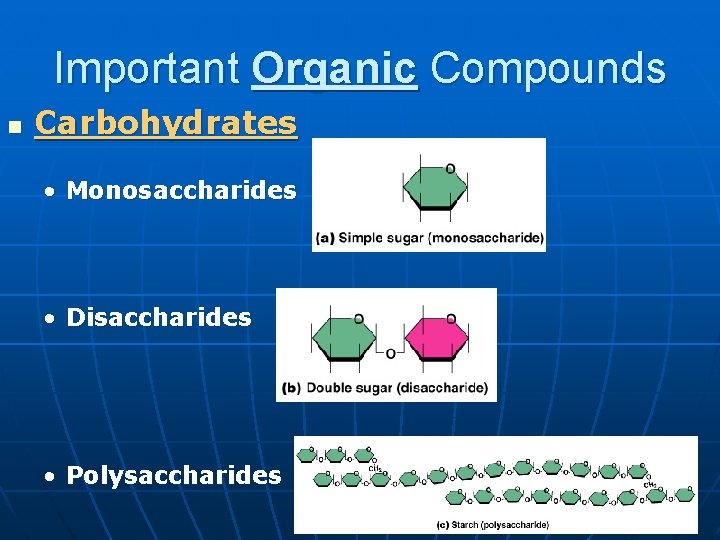
Important Organic Compounds n Carbohydrates • Monosaccharides • Disaccharides • Polysaccharides
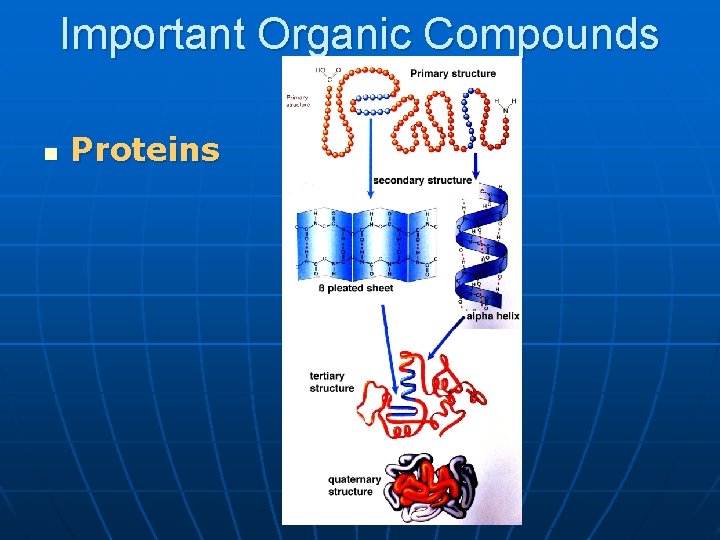
Important Organic Compounds n Proteins
Proteins n n Account for over half of the body’s organic matter Functions: • Fibrous (structural) – collagen, keratin • Globular (communication) – antibodies, hormones • Catalysts – Enzymes • Membrane Transport – protein channels

Important Organic Compounds n Lipids
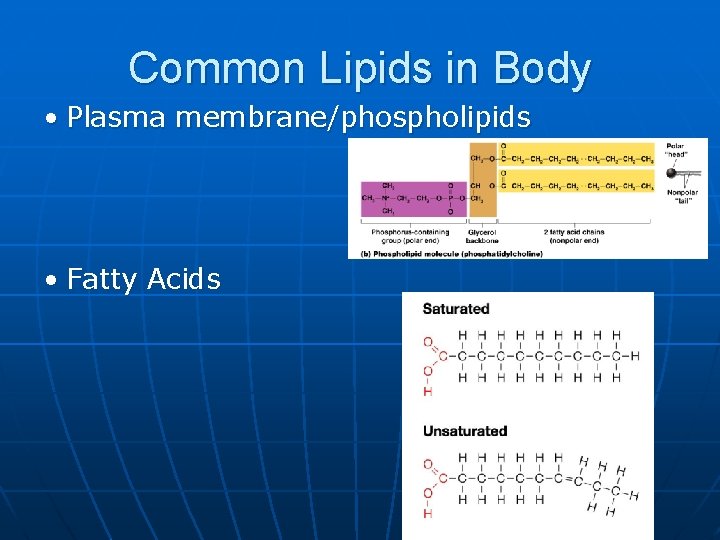
Common Lipids in Body • Plasma membrane/phospholipids • Fatty Acids
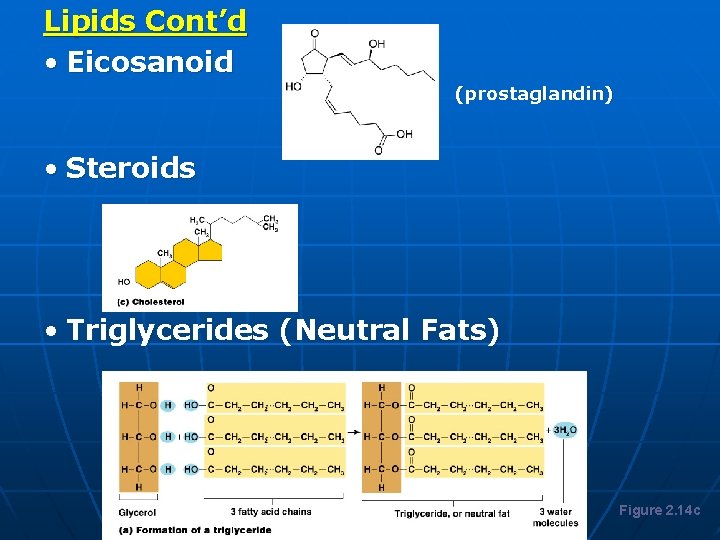
Lipids Cont’d • Eicosanoid Lipids (prostaglandin) • Steroids • Triglycerides (Neutral Fats) Figure 2. 14 c
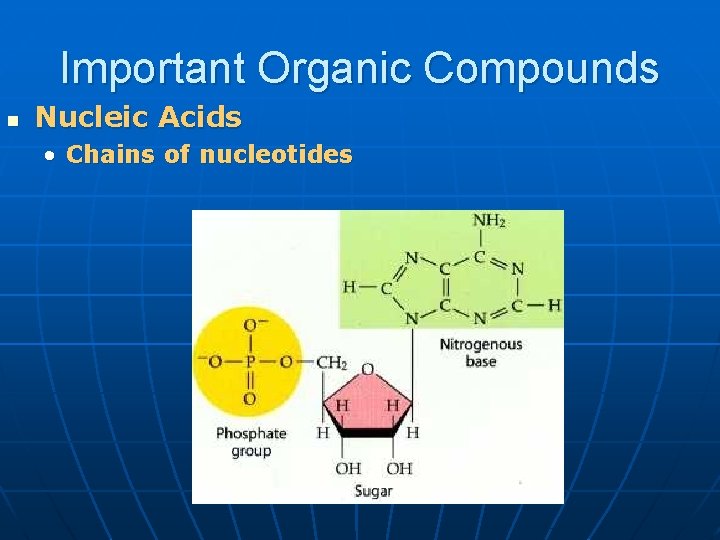
Important Organic Compounds n Nucleic Acids • Chains of nucleotides
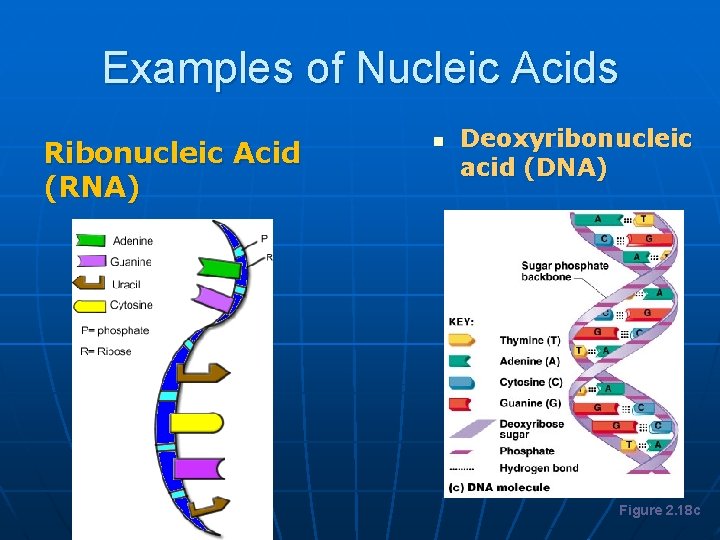
Examples of Nucleic Acids Ribonucleic Acid (RNA) n Deoxyribonucleic acid (DNA) Figure 2. 18 c
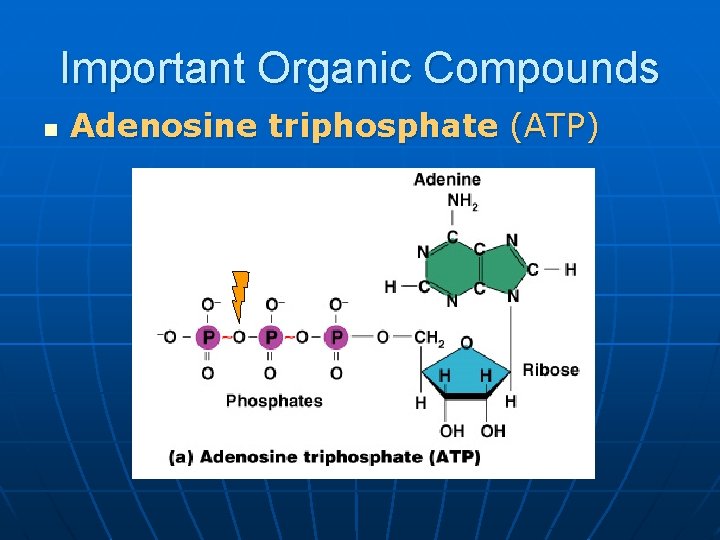
Important Organic Compounds n Adenosine triphosphate (ATP)
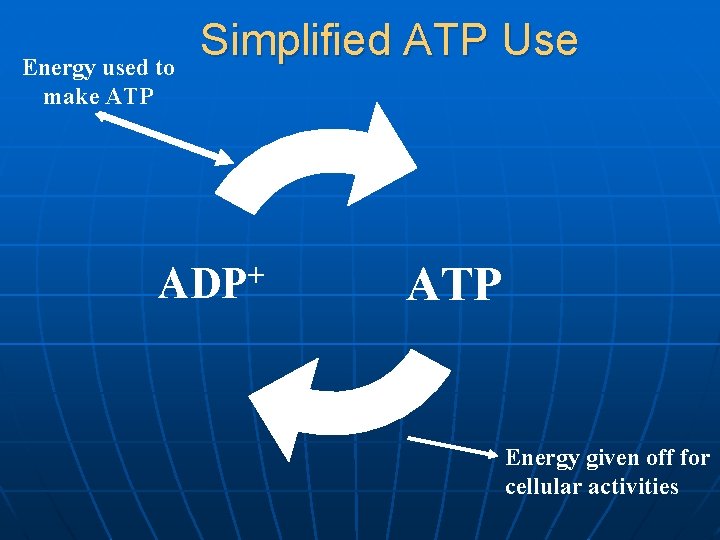
Energy used to make ATP Simplified ATP Use ADP+ ATP Energy given off for cellular activities
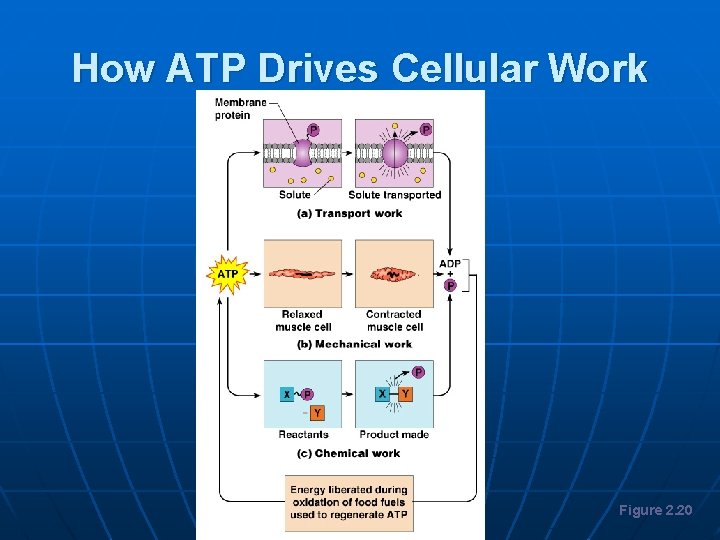
How ATP Drives Cellular Work Figure 2. 20

Quick Write Name the 5 Organic compounds and an example of them in the body: 1. 2. 3. 4. 5.
- Slides: 20