Biochemistry Elements and Compounds Element collection of one
Biochemistry







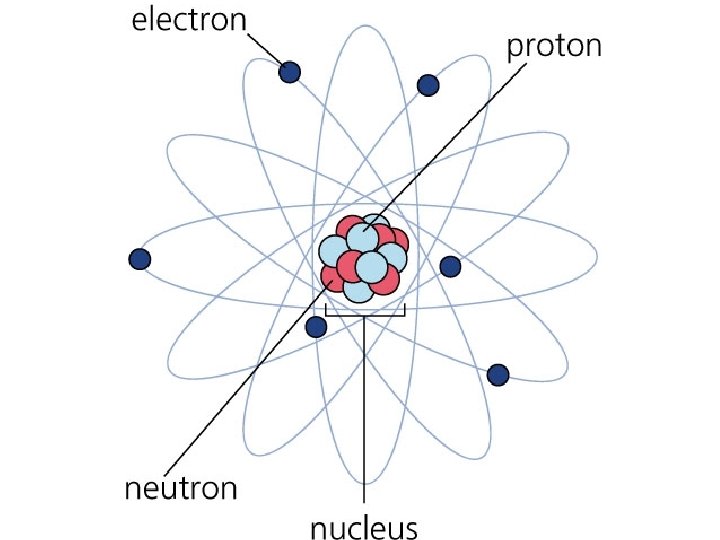

Elements and Compounds • Element – collection of one type of atom • Iron, helium, sodium, etc • Compound – two or more atoms bonded together • Na. Cl, H 2 O, CO 2

Essential Elements • • • Oxygen Carbon Hydrogen Nitrogen Phosphorus Sulfur Calcium Potassium Trace elements (iodine, iron, etc)
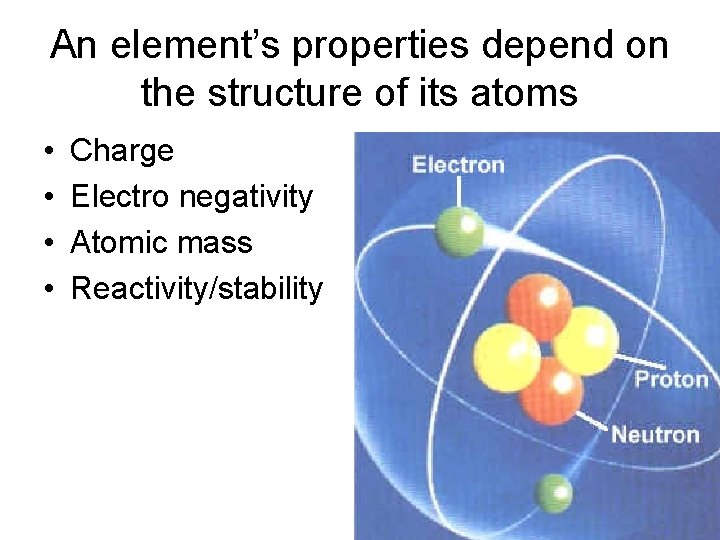
An element’s properties depend on the structure of its atoms • • Charge Electro negativity Atomic mass Reactivity/stability
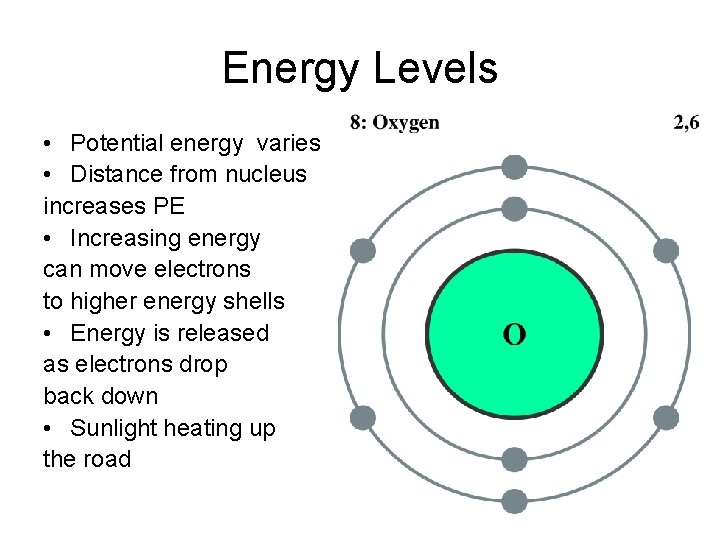
Energy Levels • Potential energy varies • Distance from nucleus increases PE • Increasing energy can move electrons to higher energy shells • Energy is released as electrons drop back down • Sunlight heating up the road
Building Atoms
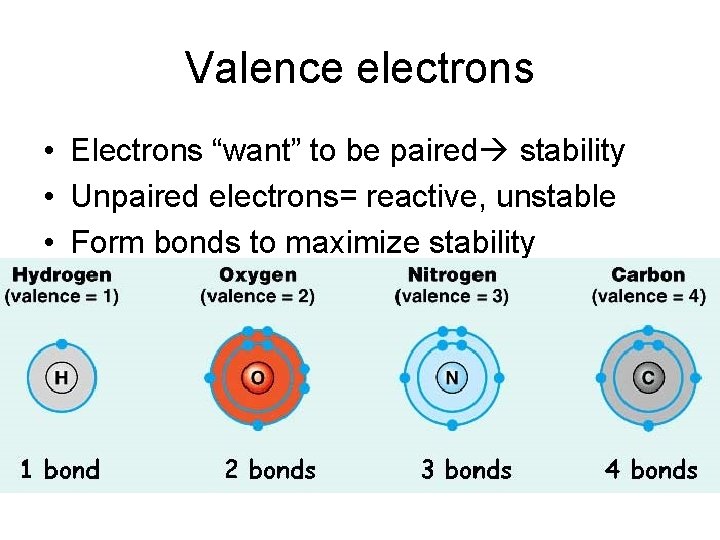
Valence electrons • Electrons “want” to be paired stability • Unpaired electrons= reactive, unstable • Form bonds to maximize stability
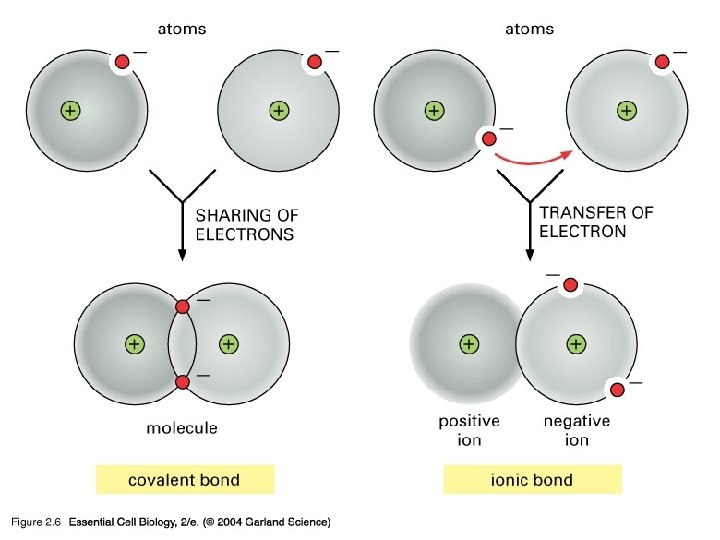
Covalent Bonds • Shared electrons • Form molecules
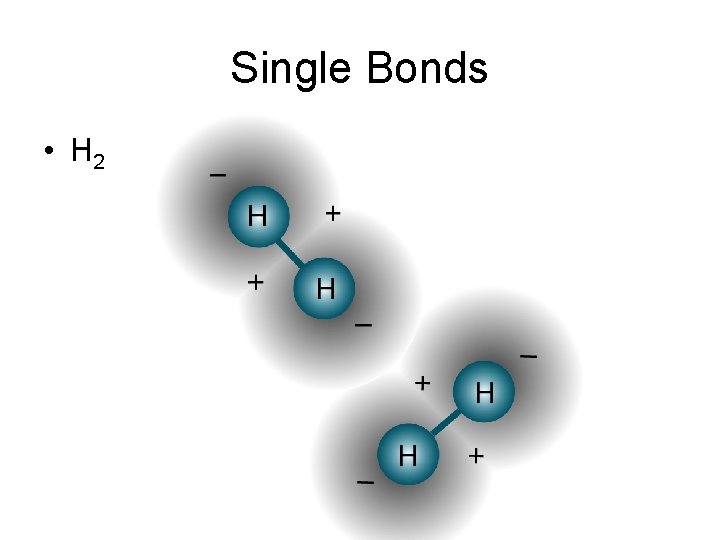
Single Bonds • H 2
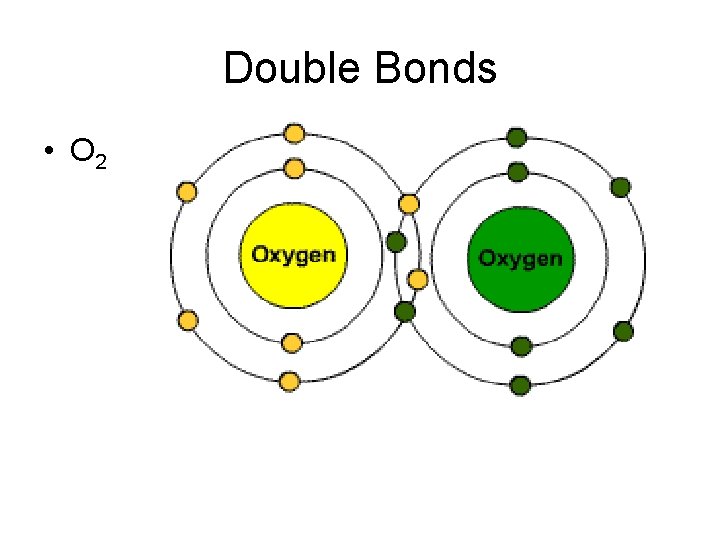
Double Bonds • O 2
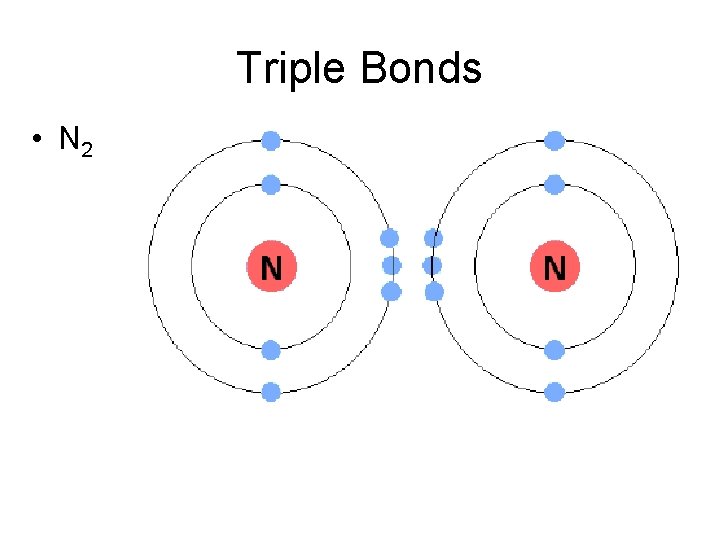
Triple Bonds • N 2
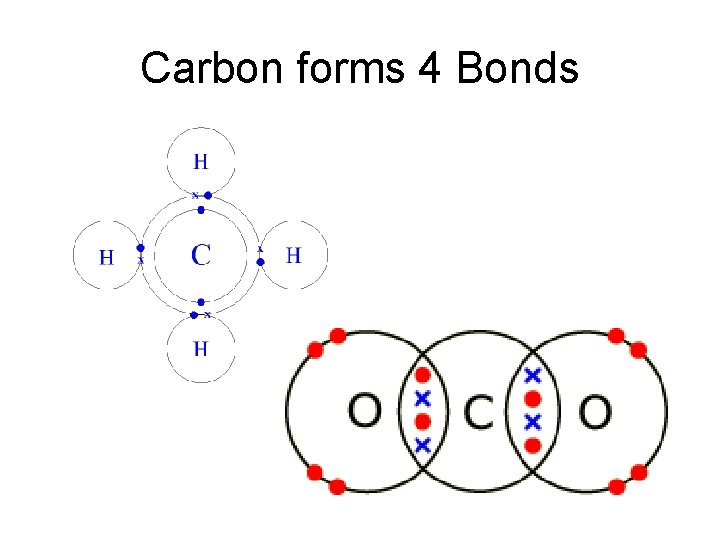
Carbon forms 4 Bonds

2 types of covalent bonds • Non-polar bonds share electrons equally – Between atoms of the same species – Create uncharged molecules • Polar bonds share electrons unequally – Between oxygen and anything else – Create slightly charged molecules
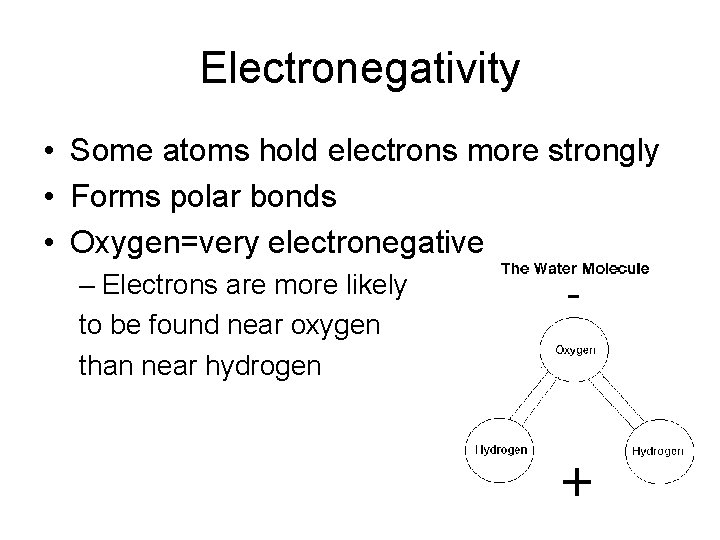
Electronegativity • Some atoms hold electrons more strongly • Forms polar bonds • Oxygen=very electronegative – Electrons are more likely to be found near oxygen than near hydrogen
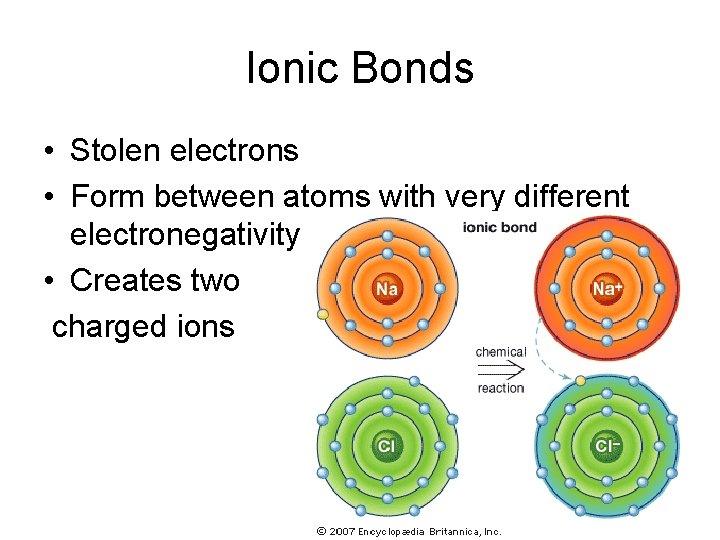
Ionic Bonds • Stolen electrons • Form between atoms with very different electronegativity • Creates two charged ions
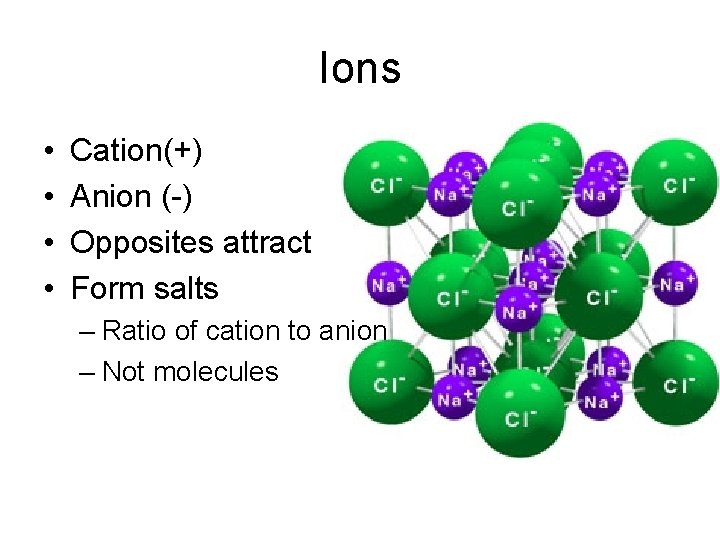
Ions • • Cation(+) Anion (-) Opposites attract Form salts – Ratio of cation to anion – Not molecules
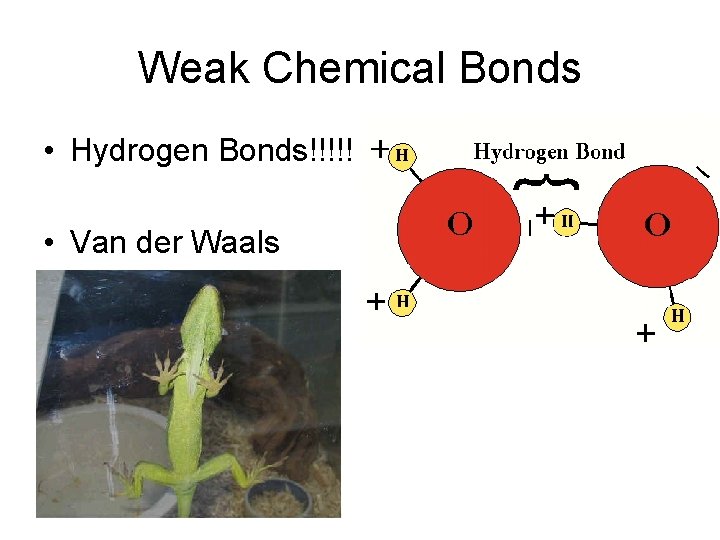
Weak Chemical Bonds • Hydrogen Bonds!!!!! • Van der Waals
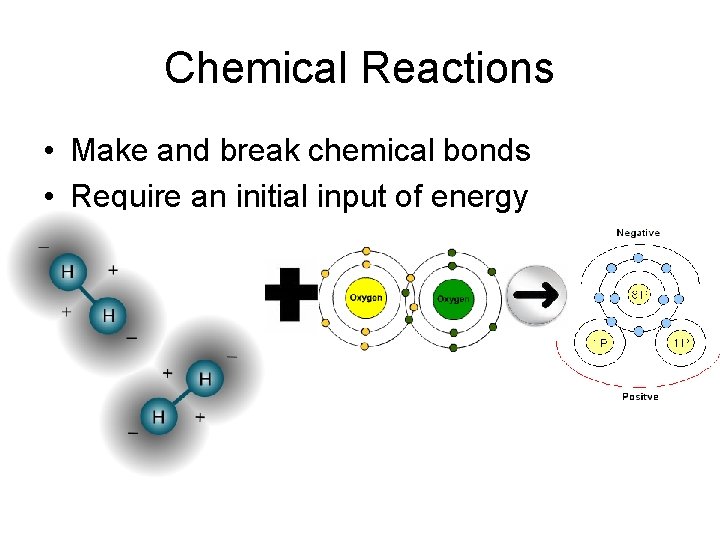
Chemical Reactions • Make and break chemical bonds • Require an initial input of energy
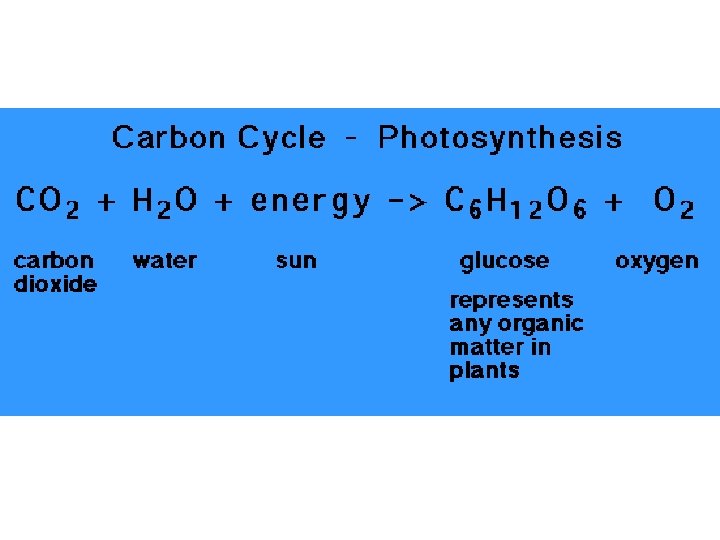
- Slides: 32