Biochemistry Chapter 3 Carbon Bonding Two categories of

Biochemistry Chapter 3

Carbon Bonding • Two categories of compounds: – Organic: made mostly of carbon • Most living things – Inorganic: mostly without carbon

Carbon Bonding • Carbon’s versatility: – Carbon atoms have 4 electrons in its outermost energy level; therefore it readily forms 4 covalent bonds – it can bond with other elements but, more importantly, with other carbons. This creates enormous variety: • • straight carbon chains branched carbon chains carbon rings double and triple bonds
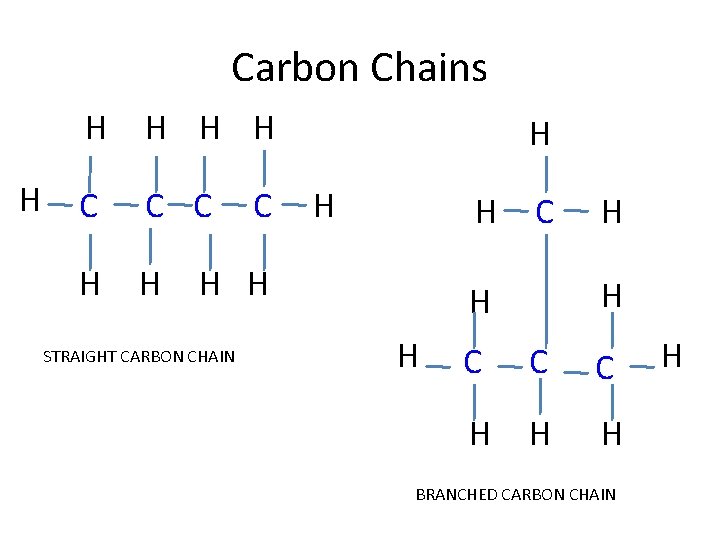
Carbon Chains H H H C C C H H H H H STRAIGHT CARBON CHAIN C H H C C C H H H BRANCHED CARBON CHAIN H
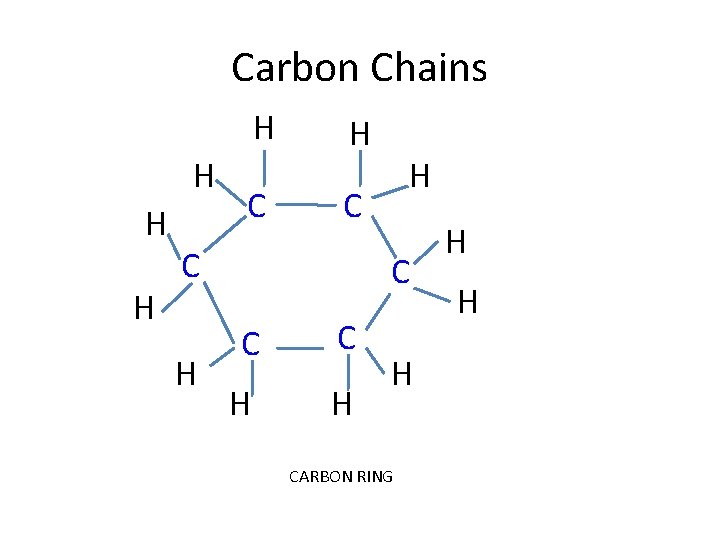
Carbon Chains H H C H C C H H C C C H H H CARBON RING H H

Single vs Double Bonds

Functional Groups: • clusters of atoms that hang together lend the molecules they attach to “personalities. ” • Refer to the table on page 52
Large Carbon Molecules – Monomers: small, simple molecules – Polymers: made up of monomers; consists of repeated, linked units
Large Carbon Molecules • Making polymers from monomers: • monomers polymers: by condensation reactions. • H+ and OH- are removed to create bonding sites. • This makes water. see p. 53
Large Carbon Molecules • Breaking polymers down into monomers: – Hydrolysis: reverse of condensation reaction – Adding water, under the right circumstances, allows for polymers to be broken down into monomers – See page 53

Energy Currency • • Adenosine Triphosphate or ATP A--P P P -- = low-energy bond; = high-energy bond P is transferred to other molecules. When the bond breaks, energy is given off to do work, like make muscles contract.
- Slides: 11