Biochemistry Biological compounds The chemistry of living organisms
Biochemistry Biological compounds The chemistry of living organisms

Chapter 20 • Read and Outline …Major headings • Pay careful attention to summary notes and terms in bold print. • KEY TERMS (you will be tested on these!)
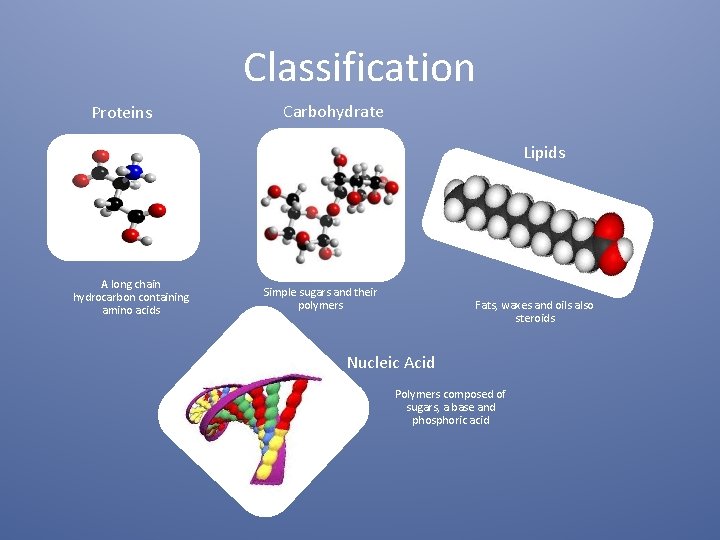
Classification Proteins Carbohydrate Lipids A long chain hydrocarbon containing amino acids Simple sugars and their polymers Fats, waxes and oils also steroids Nucleic Acid Polymers composed of sugars, a base and phosphoric acid

General Properties Important • Are they water soluble or fat soluble? – Why is this important? v Proteins, Carbohydrates and Nucleic Acids are soluble to some degree v Fats are insoluble in aqueous solution v Vitamins A, D, E, and K are fat soluble… While C, B complexes are not

Hydrocarbon Chemistry • Functional groups help us understand solubility…. – Amides (-CONH 2) lower chains are soluble larger chains and aryl-amides not so much! Acids, Aldehydes, Ketones the same… check tables in Chapter 19. Check for patterns.
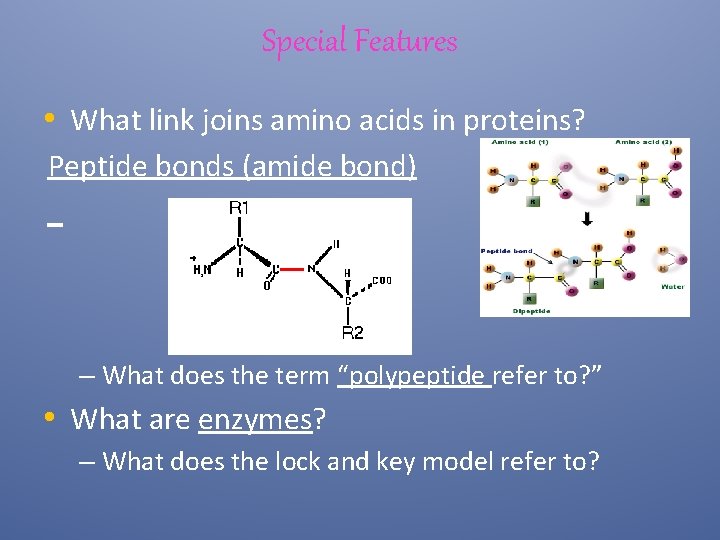
Special Features • What link joins amino acids in proteins? Peptide bonds (amide bond) – What does the term “polypeptide refer to? ” • What are enzymes? – What does the lock and key model refer to?

Carbohydrates • Usually contains an aldehyde or ketone group • Bond between sugars is called a glycoside linkage • sugar – O - sugar

Lipids Water insoluble Contains an alcohol and one or more carboxylic acid molecules Fats and oils 3 Carboxylic acids are linked by an ester and joined to a glycerol

Nucleic acid • Biochemical polymer • Contains a sugar attached to nitrogen base and phosphate group • Units linked by phosphate linkage • Base- sugar- phosphate- sugar - base
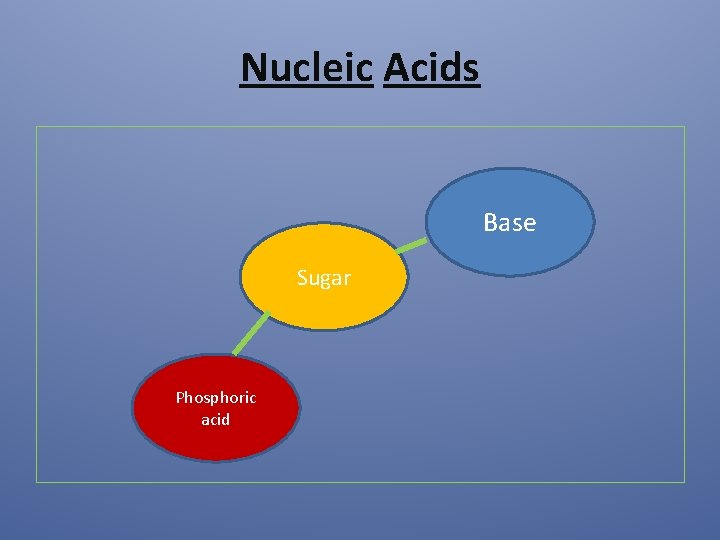
Nucleic Acids Base Sugar Phosphoric acid
Nucleic acids • • Contain genetic information DNA- deoxyribonucleic acid RNA- ribonucleic acid Different functions – only difference is a hydroxyl group (-OH)
Structure • Proteins are made up of amino acid structures that are linked together by a PEPTIDE BOND. • Proteins have a primary, secondary and tertiary structure
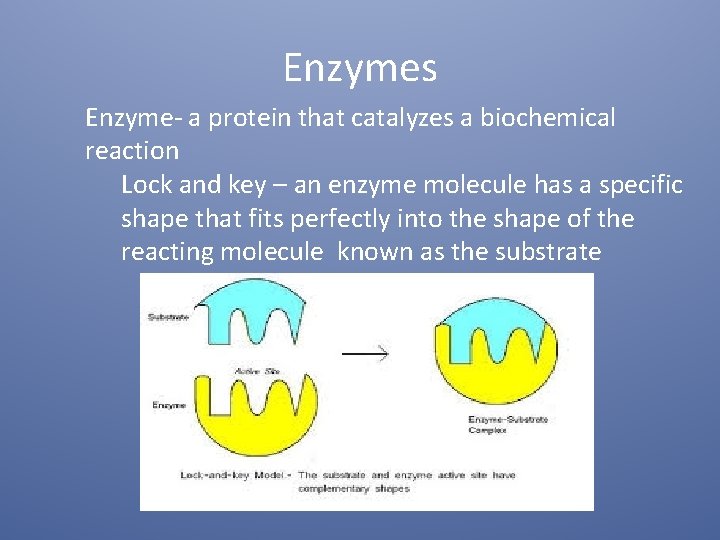
Enzymes Enzyme- a protein that catalyzes a biochemical reaction Lock and key – an enzyme molecule has a specific shape that fits perfectly into the shape of the reacting molecule known as the substrate
Nomenclature of proteins • Important features – R- group refers to the hydrocarbon side chain attached to the a–carbon. – R- groups may be neutral, acidic or basic.

Lipids § Oils…. . unsaturated (plant source) § Fats…. saturated (animal) § Waxes are lipids…think about candles and the “old days” § Steroids are lipids § -ol endings or –one ending § Read about vitamins (p. 585)

DNA and RNA • What are the differences? • What is the same?
Other Patterns v. Nomenclature v. Endings are important v. Check the endings of functional groups… v. Ex: R-OH alcohol, ending –ol v. Ex: R-CHO aldehyde, ending –al v. Lets try # 93, 94, 95 (p. 566) v. How about the biomolecules…. v. Make a list for yourself to study…
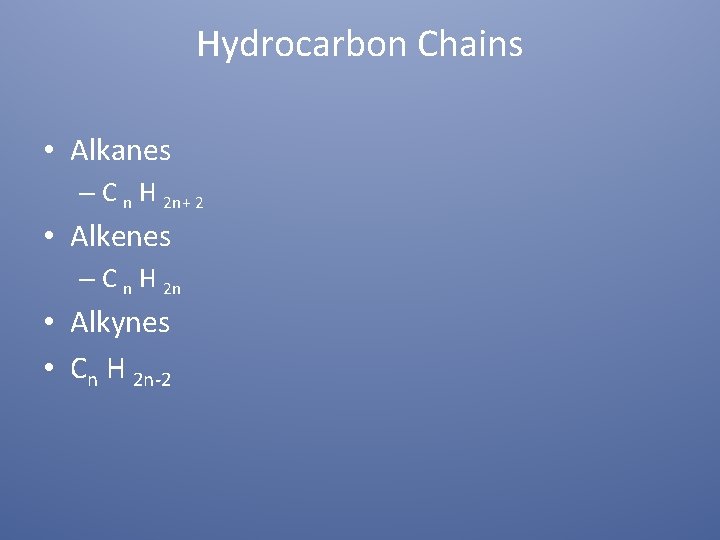
Hydrocarbon Chains • Alkanes – C n H 2 n+ 2 • Alkenes – C n H 2 n • Alkynes • Cn H 2 n-2
Check out the Patterns • Name longest consecutive chain • Prefixes – – – – – Meth Eth Prop But Pent Hex Hept Oct Non Dec

Alkanes • Carbon in chain contains all single bonds • Must be four bonds around each Carbon • Saturated compounds

Alkenes • Unsaturated (contains double bond…at least one) • Carbon with lowest number between double bonds names the bond.

Alkynes § § Carbon chain contains a triple bond Acetylene lowest chain Highly unsaturated Triple bonds > double >single in bond strength § Makes chemical properties different
Isomers • Carbon chain with same molecular formula but different structural formula

Look for patterns in the names • How are carbohydrates named? • What about steroids that are hormones? – What family of biochemistry do steroids belong to? – What is the general form of a fatty acid? – Try # 1 and 2 on p. 591!
- Slides: 24