Biochemistry A Connection Between Life and Chemistry What
Biochemistry A Connection Between Life and Chemistry
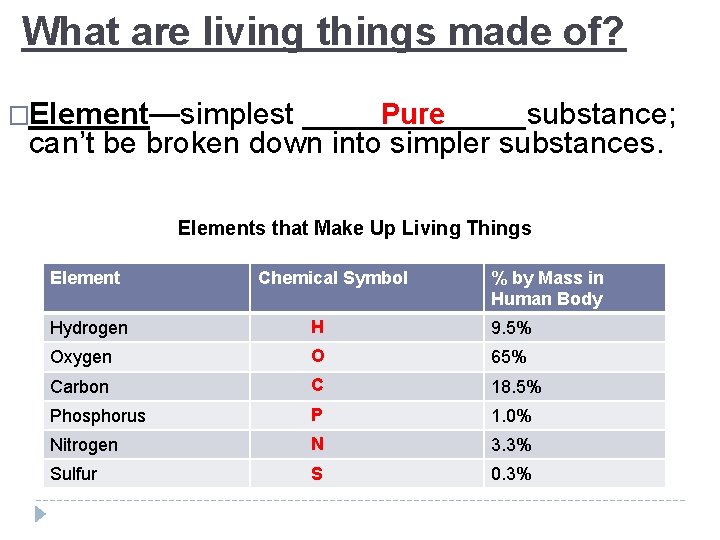
What are living things made of? �Element—simplest _______substance; Pure can’t be broken down into simpler substances. Elements that Make Up Living Things Element Chemical Symbol % by Mass in Human Body Hydrogen H 9. 5% Oxygen O 65% Carbon C 18. 5% Phosphorus P 1. 0% Nitrogen N 3. 3% Sulfur S 0. 3%
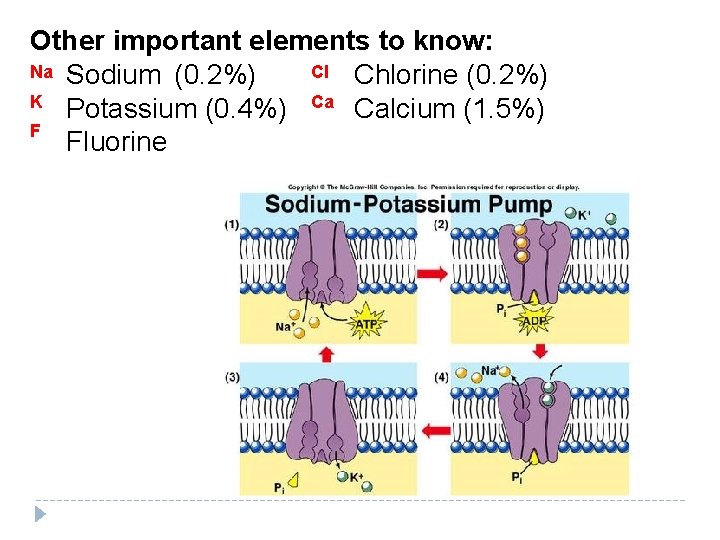
Other important elements to know: Na Sodium (0. 2%) Cl Chlorine (0. 2%) K Potassium (0. 4%) Ca Calcium (1. 5%) F Fluorine
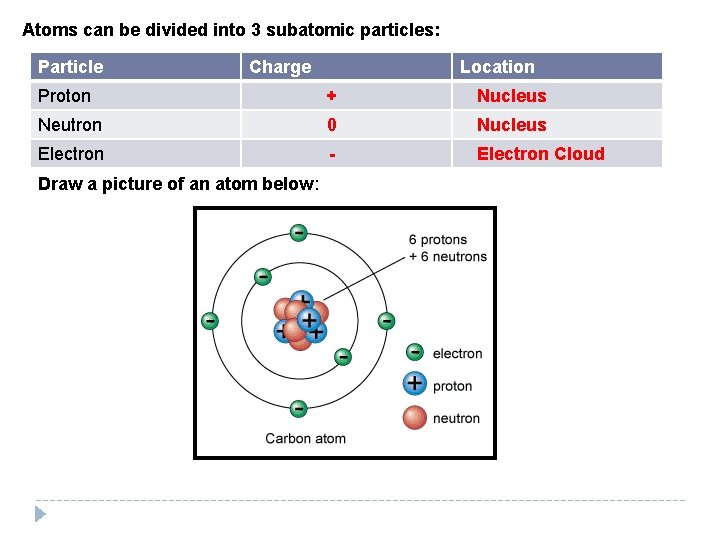
Atoms can be divided into 3 subatomic particles: Particle Charge Location Proton + Nucleus Neutron 0 Nucleus Electron - Electron Cloud Draw a picture of an atom below:
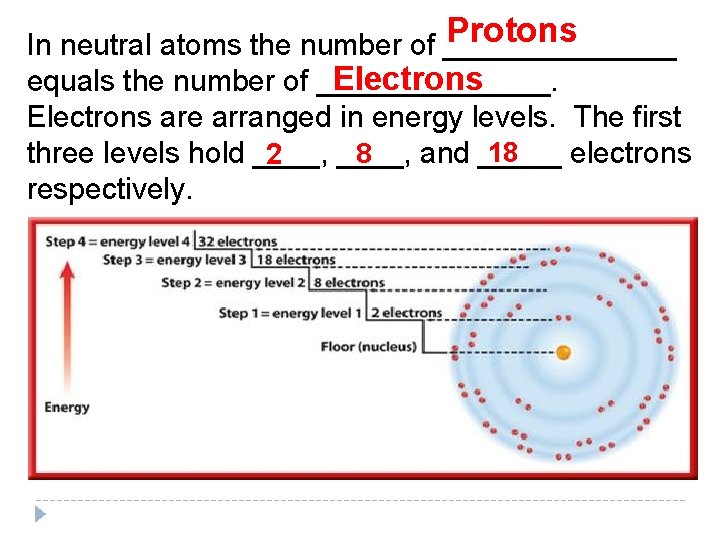
Protons In neutral atoms the number of _______ Electrons equals the number of _______. Electrons are arranged in energy levels. The first 18 8 three levels hold ____, and _____ electrons 2 respectively.
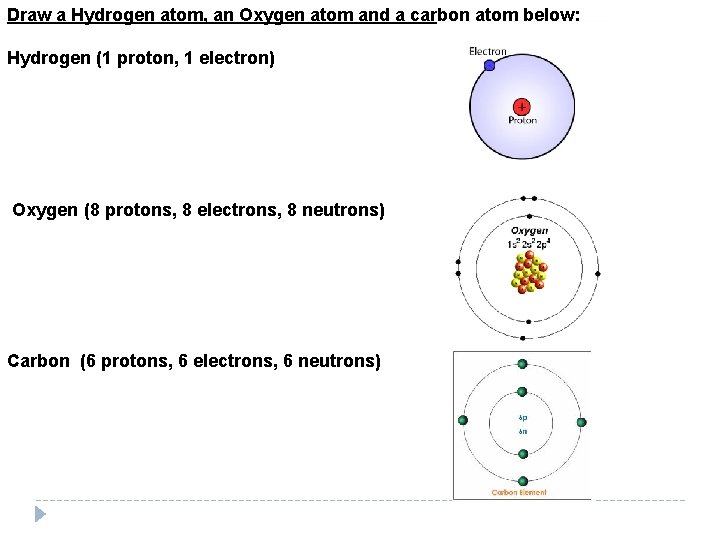
Draw a Hydrogen atom, an Oxygen atom and a carbon atom below: Hydrogen (1 proton, 1 electron) Oxygen (8 protons, 8 electrons, 8 neutrons) Carbon (6 protons, 6 electrons, 6 neutrons)
Atoms of 2 or more different elements can be ___________ joined together to form Chemically compounds, which have properties different from those of the individual elements. C 6 H 12 O 6 Examples: H 2 O Na. Cl There are 2 types of compounds: Organic Compounds— Compounds containing Carbon (Living) that do NOT contain Inorganic Compounds— Compounds Carbon (Non-living)
Mixture—a combination of elements that are not ________ combined. The mixture has Chemically properties similar to the elements that make it up. Examples: Sugar Water Blood (mix of salt, H 2 O, and cells) A chemical formula is the symbolic representation of a Compound Molecule _______ or _______. Example: H 2 O Elements: H- Hydrogen= 2 atoms O- Oxygen= 1 atom Atoms Subscript--tells how many ________ of a particular element are used in the compound. If there is no number written, it is assumed to be 1). Example: KMn. O 4 Elements: K- 1 Potassium Permanganate Mn- 1 O- 4
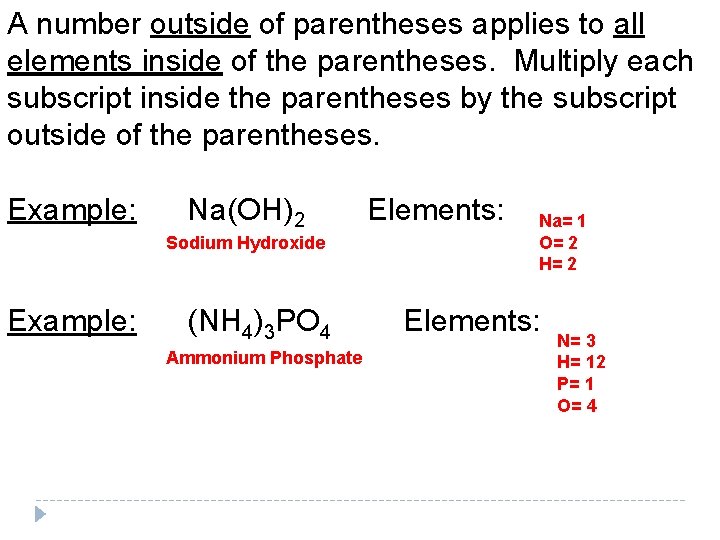
A number outside of parentheses applies to all elements inside of the parentheses. Multiply each subscript inside the parentheses by the subscript outside of the parentheses. Example: Na(OH)2 Sodium Hydroxide (NH 4)3 PO 4 Ammonium Phosphate Elements: Na= 1 O= 2 H= 2 Elements: N= 3 H= 12 P= 1 O= 4
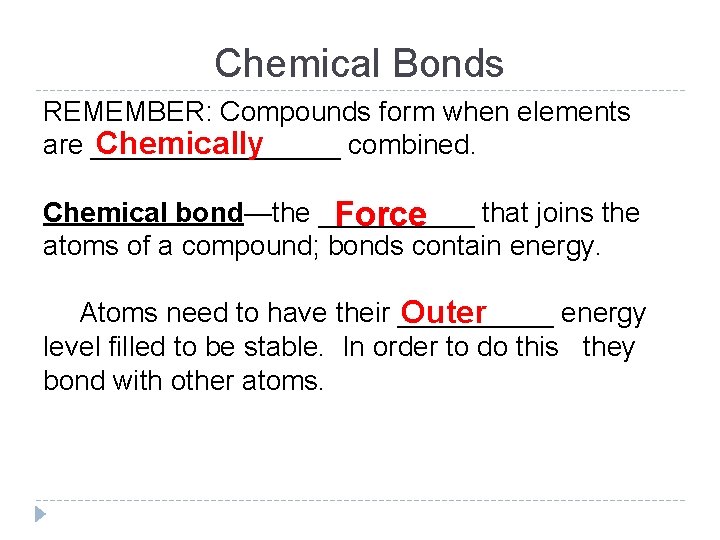
Chemical Bonds REMEMBER: Compounds form when elements Chemically are ________ combined. Chemical bond—the _____ that joins the Force atoms of a compound; bonds contain energy. Atoms need to have their _____ energy Outer level filled to be stable. In order to do this they bond with other atoms.
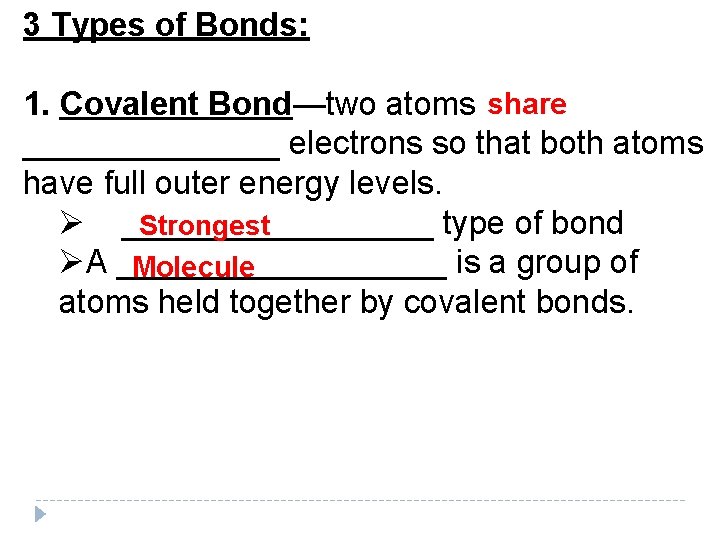
3 Types of Bonds: 1. Covalent Bond—two atoms share _______ electrons so that both atoms have full outer energy levels. Ø _________ type of bond Strongest ØA _________ is a group of Molecule atoms held together by covalent bonds.
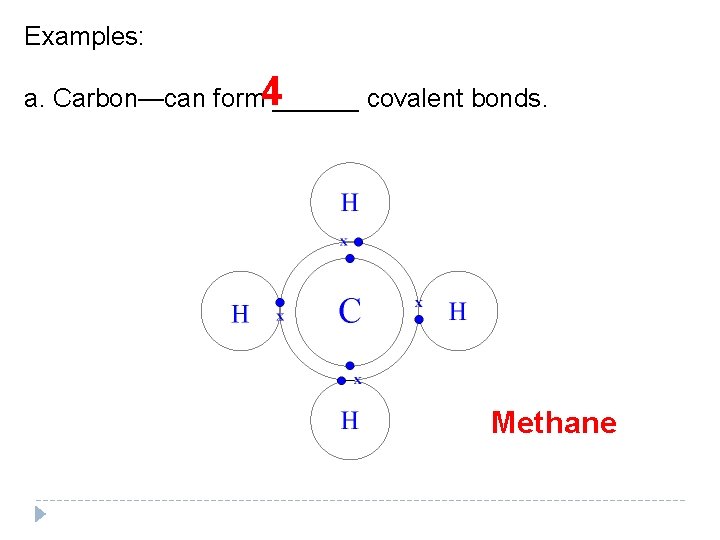
Examples: 4 a. Carbon—can form ______ covalent bonds. Methane
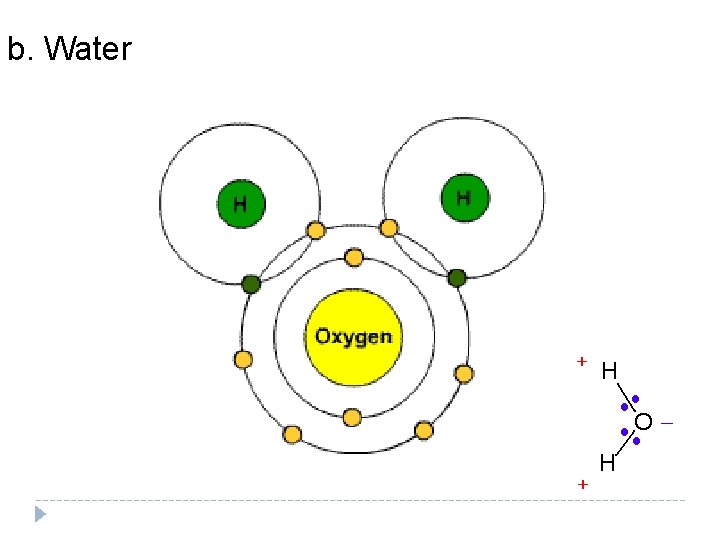
b. Water

2. Ionic Bond—electrons are ________ from one atom to another so Transferred that both atoms have full outer energy levels. One atom loses electrons and becomes ____ + charged. The other atom gains electrons and becomes ___ charged. Why? ion A charged atom is called an _____. Water Ionic bonds break apart in ________.
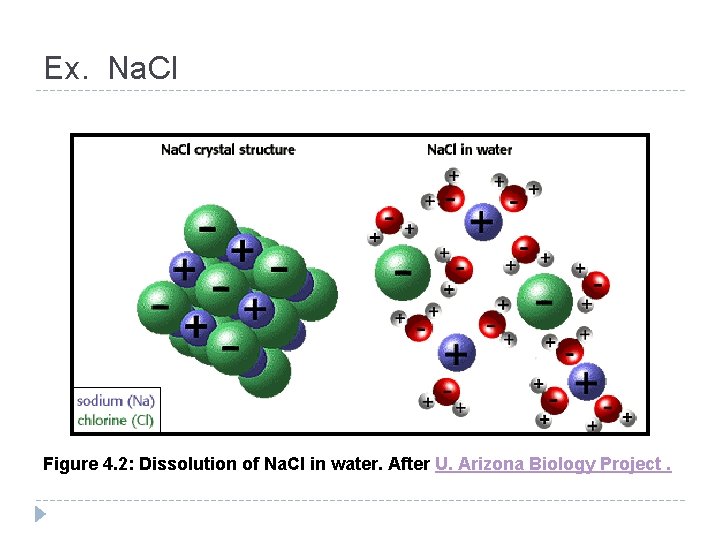
Ex. Na. Cl Figure 4. 2: Dissolution of Na. Cl in water. After U. Arizona Biology Project.

Properties of Water Remember, so far we have talked about 2 types of chemical bonds. 1. Covalent Bonds 2. Ionic Bonds
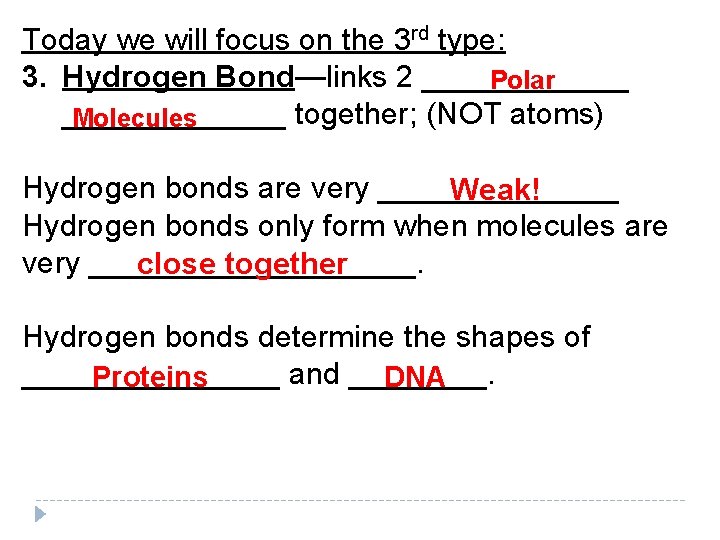
Today we will focus on the 3 rd type: 3. Hydrogen Bond—links 2 ______ Polar _______ together; (NOT atoms) Molecules Hydrogen bonds are very _______ Weak! Hydrogen bonds only form when molecules are very __________. close together Hydrogen bonds determine the shapes of ________ and ____. Proteins DNA
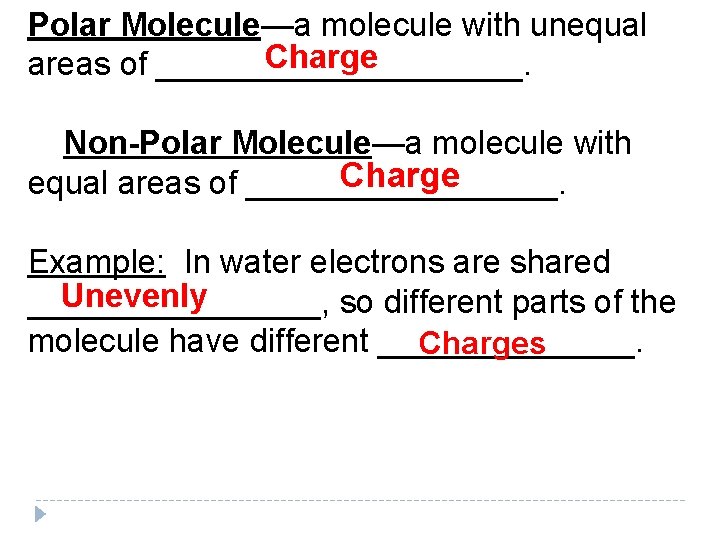
Polar Molecule—a molecule with unequal Charge areas of __________. Non-Polar Molecule—a molecule with Charge equal areas of _________. Example: In water electrons are shared Unevenly ________, so different parts of the molecule have different _______. Charges
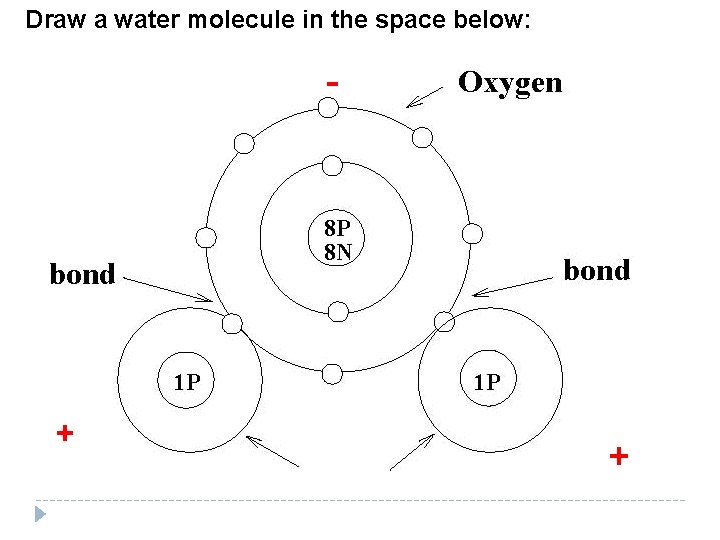
Draw a water molecule in the space below: - + +
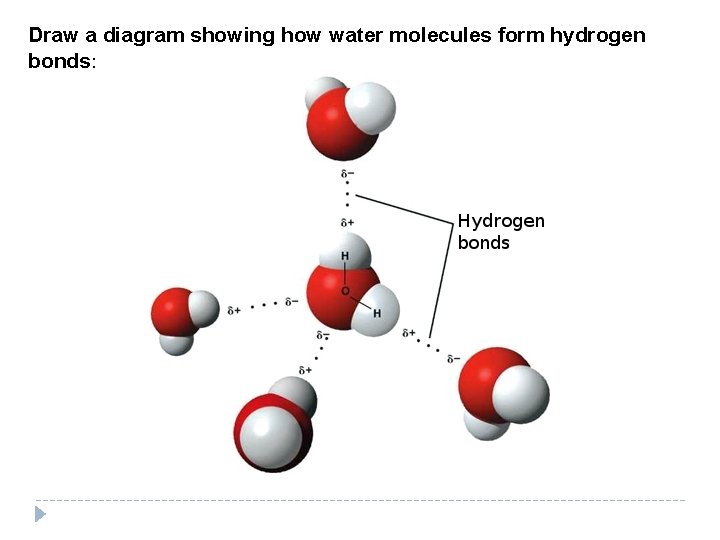
Draw a diagram showing how water molecules form hydrogen bonds:

Other Properties of Water: Cohesion— Attraction between water molecules (creates surface tension). Water forms drops. Example: Adhesion— Attraction of water molecules to another polar molecule. Example: Water sticking to glass! Water is called the _____________; many universal solvent things can dissolve in water because it is a polar molecule. Solvent = A substance in which something can be dissolved!
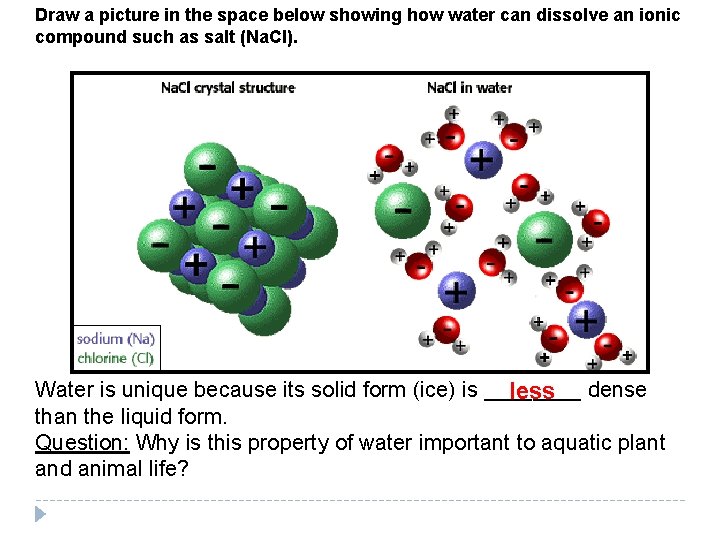
Draw a picture in the space below showing how water can dissolve an ionic compound such as salt (Na. Cl). Water is unique because its solid form (ice) is ____ dense less than the liquid form. Question: Why is this property of water important to aquatic plant and animal life?
Macromolecules The study of compounds that contain bonds between carbon atoms is called _________ chemistry. Organic Macromolecule--______ molecule. There are 4 main Large groups of biological macromolecules (biomolecules). Macromolecules are made by linking smaller molecules called ___________ to form _______. Monomer Polymers Monomers are joined together through a chemical process called dehydration synthesis. Polymers can be broken down into their component parts through a chemical process called hydrolysis.
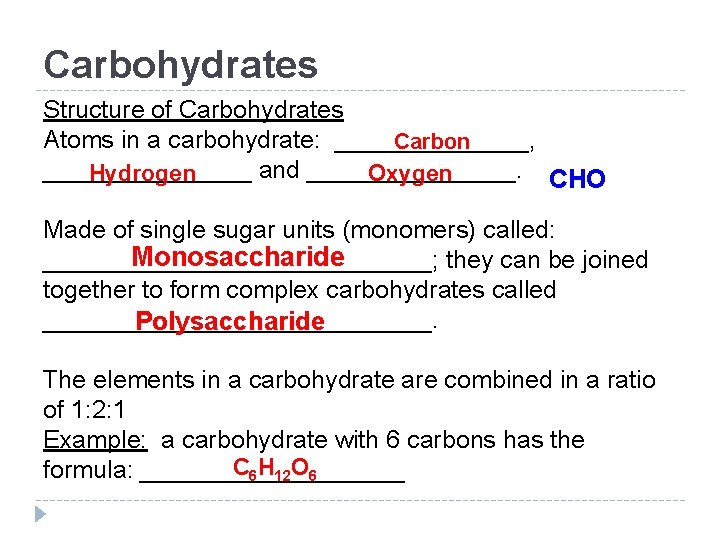
Carbohydrates Structure of Carbohydrates Atoms in a carbohydrate: _______, Carbon ________ and ________. Hydrogen Oxygen CHO Made of single sugar units (monomers) called: Monosaccharide ______________; they can be joined together to form complex carbohydrates called ______________. Polysaccharide The elements in a carbohydrate are combined in a ratio of 1: 2: 1 Example: a carbohydrate with 6 carbons has the C 6 H 12 O 6 formula: __________
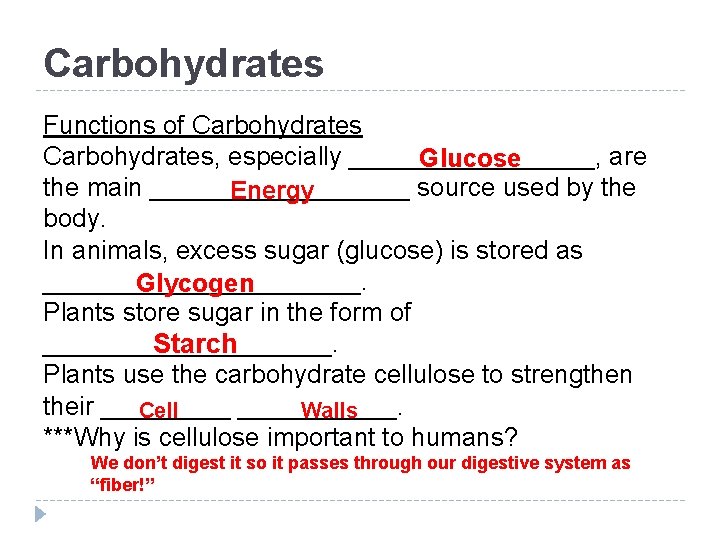
Carbohydrates Functions of Carbohydrates, especially _________, are Glucose the main _________ source used by the Energy body. In animals, excess sugar (glucose) is stored as ___________. Glycogen Plants store sugar in the form of __________. Starch Plants use the carbohydrate cellulose to strengthen their ___________. Cell Walls ***Why is cellulose important to humans? We don’t digest it so it passes through our digestive system as “fiber!”
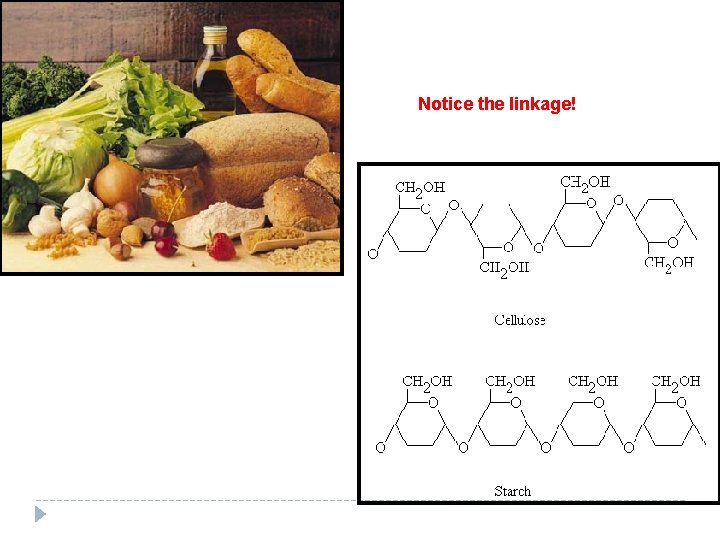
Notice the linkage!
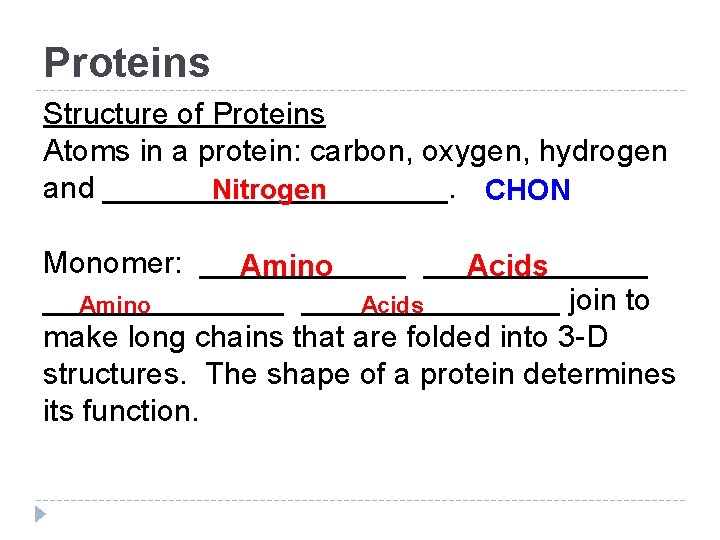
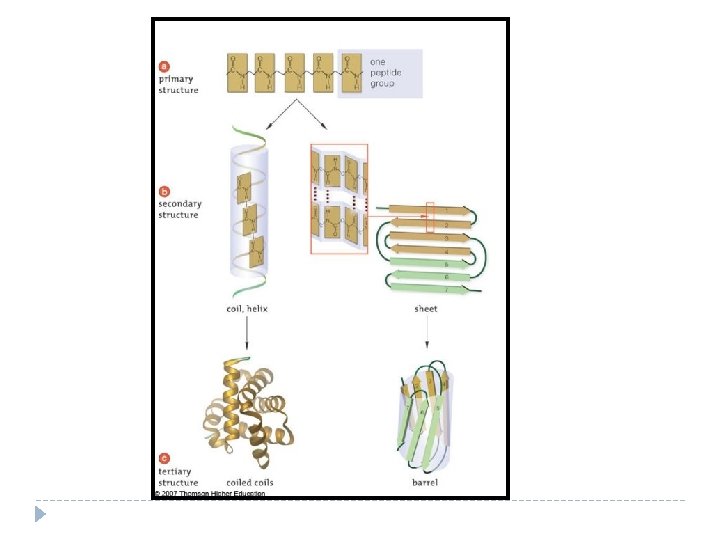
Proteins Structure of Proteins Atoms in a protein: carbon, oxygen, hydrogen and __________. Nitrogen CHON Monomer: _____________ Amino Acids _______________ join to Amino Acids make long chains that are folded into 3 -D structures. The shape of a protein determines its function.
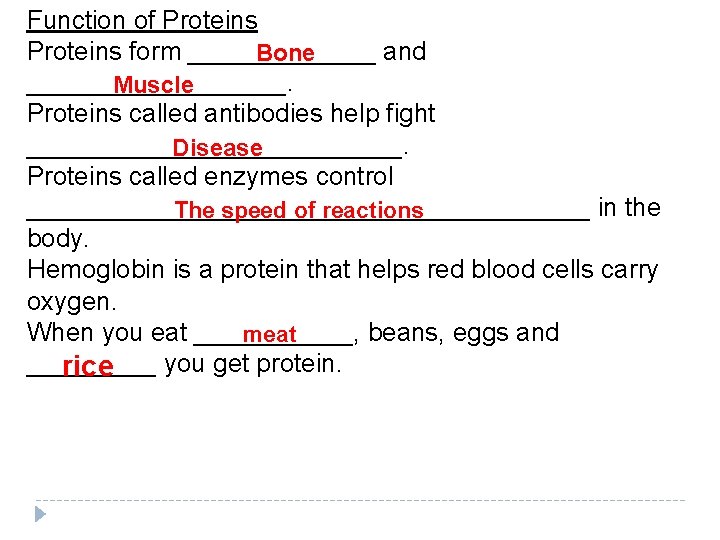
Function of Proteins form _______ and Bone _________. Muscle Proteins called antibodies help fight _____________. Disease Proteins called enzymes control ____________________ in the The speed of reactions body. Hemoglobin is a protein that helps red blood cells carry oxygen. When you eat ______, beans, eggs and meat _____ you get protein. rice

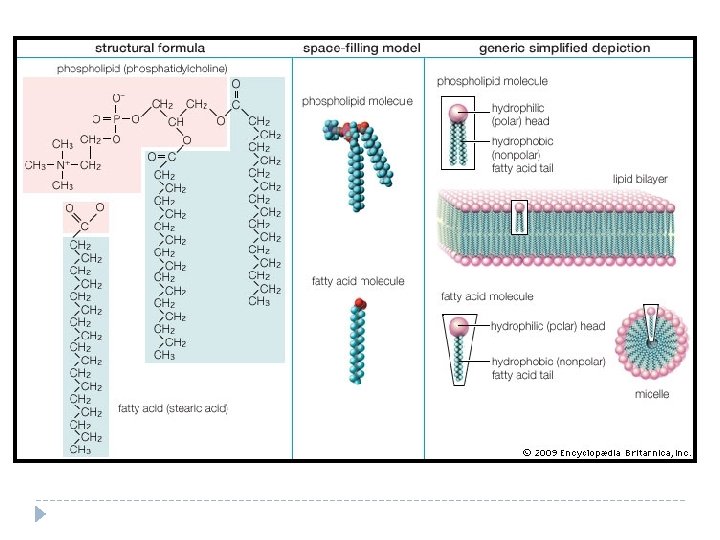
Lipids Structure of Lipids are not __________ in water. Soluble This is because water is polar and most lipids are nonpolar. *****Use your knowledge of polar and nonpolar substances to explain why lipids are unable to dissolve in a polar substance such as water? Because lipids are nonpolar they are not attracted to either end of the H 2 O molecule, and therefore dissociation does not occur.
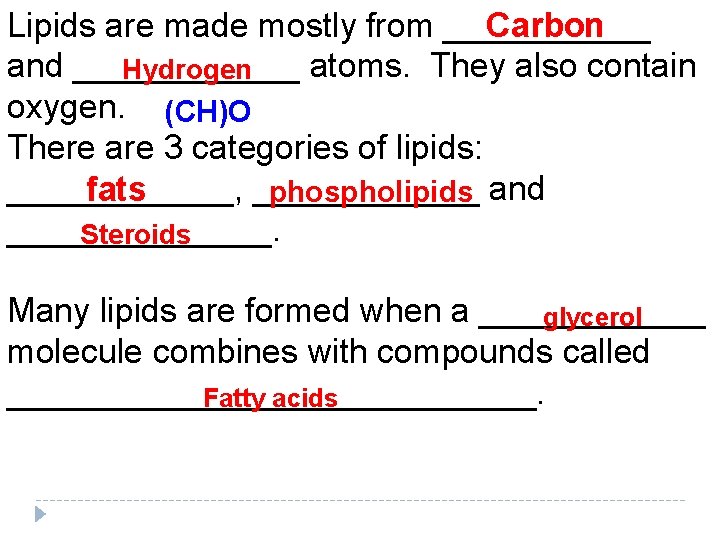
Carbon Lipids are made mostly from ______ and ______ atoms. They also contain Hydrogen oxygen. (CH)O There are 3 categories of lipids: ______, ______ and fats phospholipids _______. Steroids Many lipids are formed when a ______ glycerol molecule combines with compounds called ______________. Fatty acids
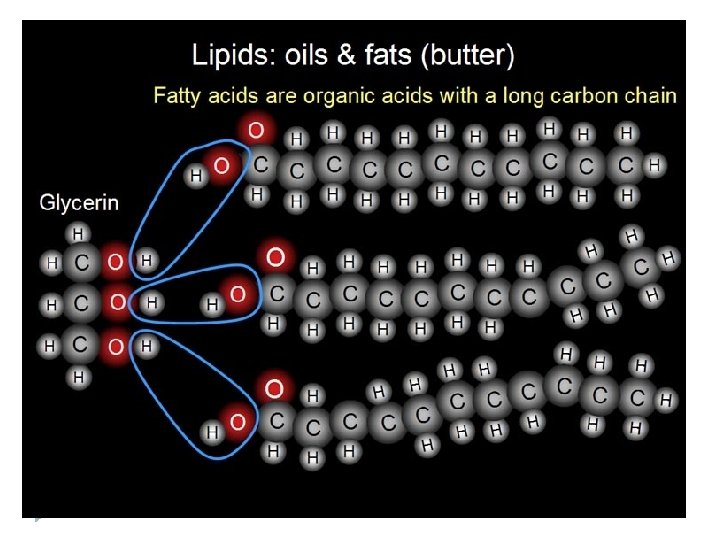

Function of Lipids cook food Lipids can be used to _____. Lipids are important parts of ____ Cell ______ and ___________ Membrane Leaf (wax) coverings. Lipids also form steroids which serve as _________ messengers in the body. chemical
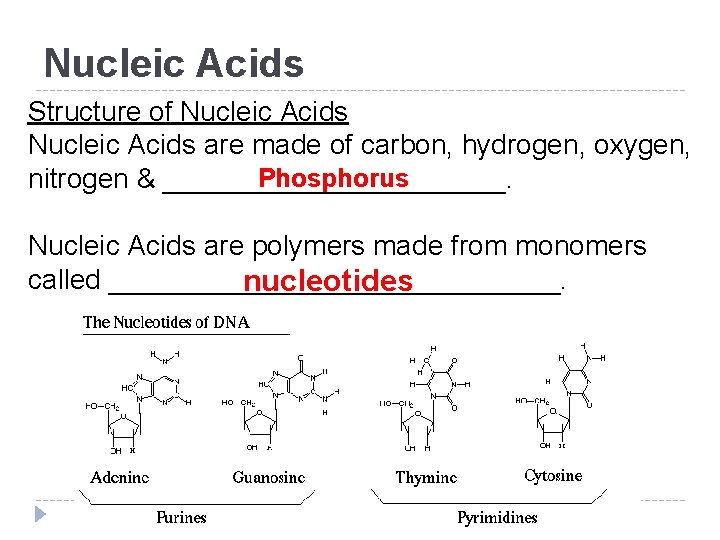
Nucleic Acids Structure of Nucleic Acids are made of carbon, hydrogen, oxygen, Phosphorus nitrogen & ___________. Nucleic Acids are polymers made from monomers called _______________. nucleotides
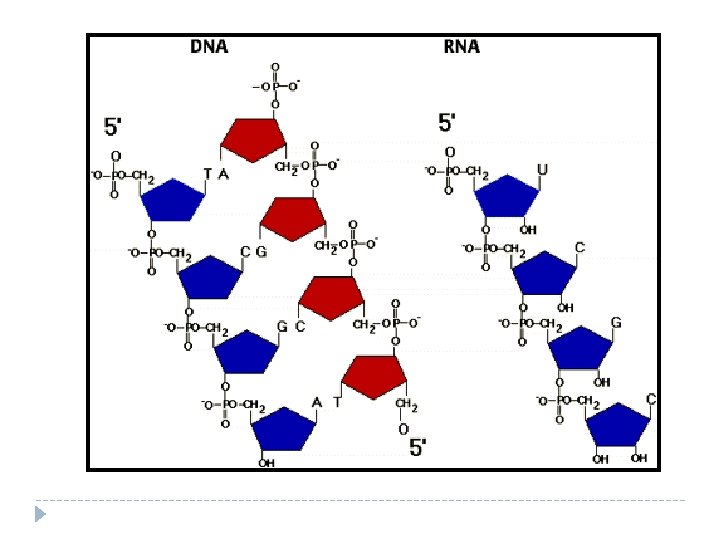
Function of Nucleic Acids Nucleic acids store and transmit ________, or _______ Genetic Genes information. There are 2 kinds of nucleic acids: ______________ (RNA) Ribonucleic Acids and __________________ Deoxyribonucleic Acids (DNA).
1. Based only on chemical structure, how could you tell carbohydrates, proteins and nucleic acids apart? 2. What elements do all 4 macromolecules have in common? 3. Describe one function of each group of macromolecules.
- Slides: 38