Biochemistry 10412 II Protein Structure H Protein folding
Biochemistry 10/4/12 II. Protein Structure H. Protein folding Thermodynamics & Kinetics Chaperones Neurodegenerative Disease Assignment #9 for 10/9/12 Read Chapter 13 (13. 1 -13. 3) Finish Problem Set 3 .
Protein stability • Native state only marginally more stable than denatured state • Contributions to protein stability – hydrophobic effect: entropic effect – hydrogen bonds: net effect = 0 – others • • salt bridges disulphide bonds aromatic-aromatic interactions metal binding
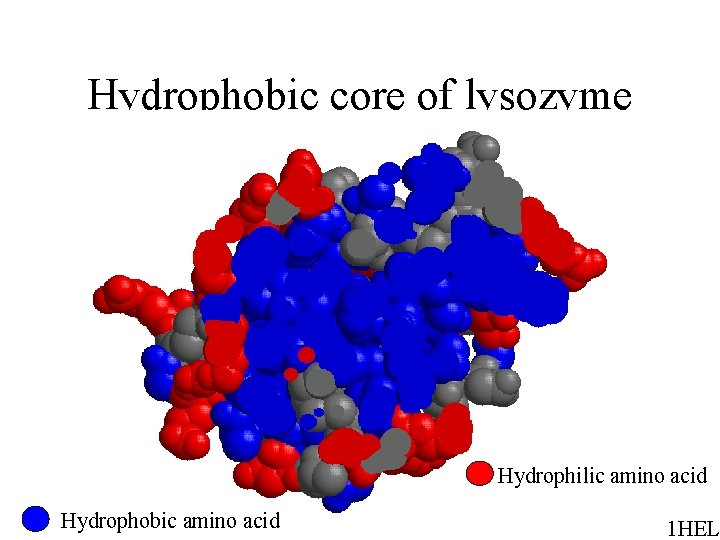
Hydrophobic core of lysozyme Hydrophilic amino acid Hydrophobic amino acid 1 HEL

Escherichia coli
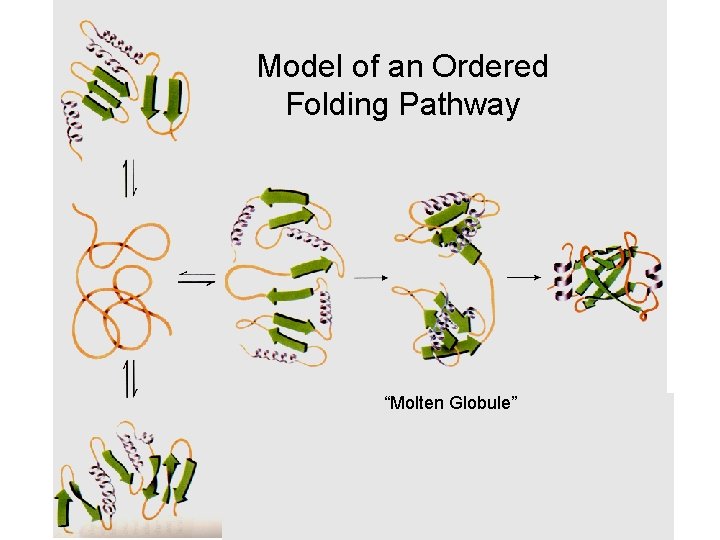
Model of an Ordered Folding Pathway “Molten Globule”
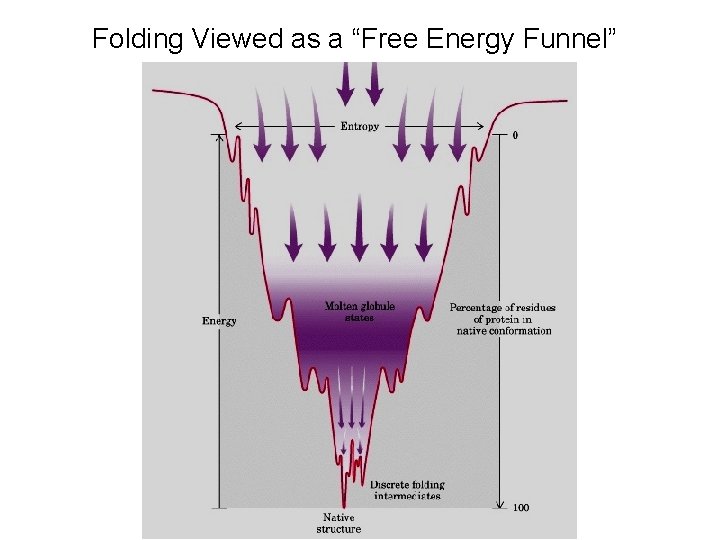
Folding Viewed as a “Free Energy Funnel”
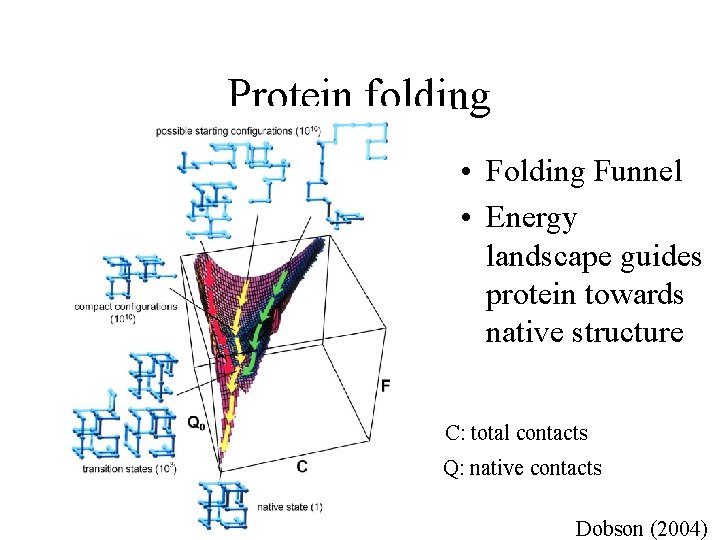
Protein folding • Folding Funnel • Energy landscape guides protein towards native structure C: total contacts Q: native contacts Dobson (2004)
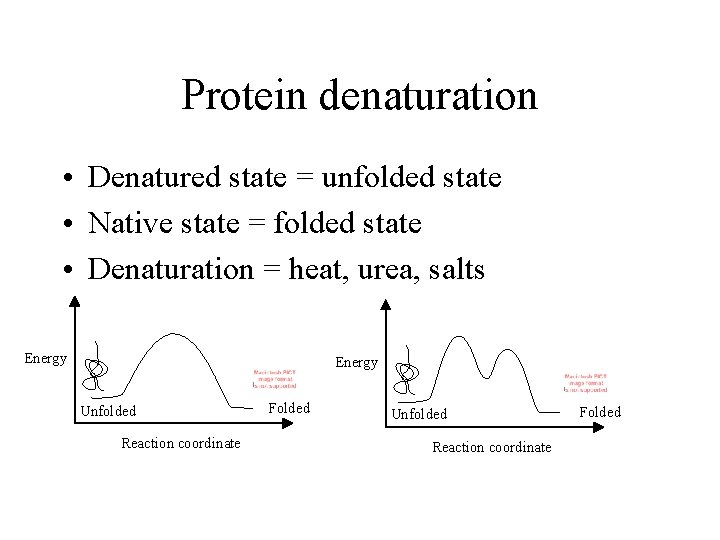
Protein denaturation • Denatured state = unfolded state • Native state = folded state • Denaturation = heat, urea, salts Energy Unfolded Reaction coordinate Folded
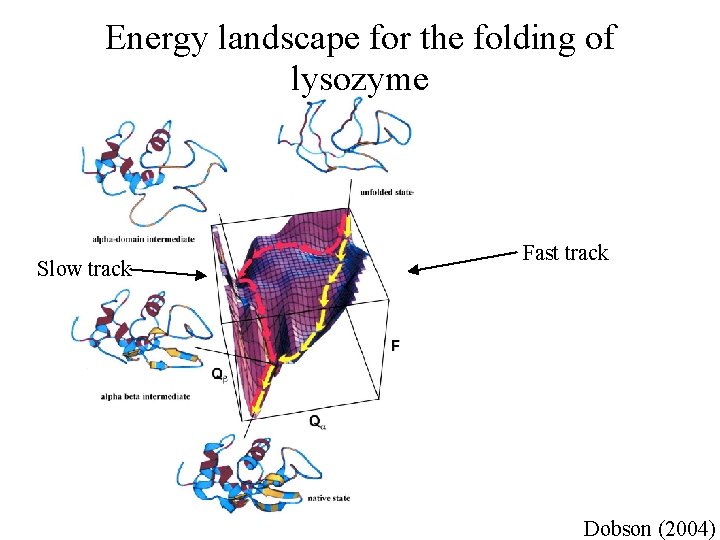
Energy landscape for the folding of lysozyme Slow track Fast track Dobson (2004)
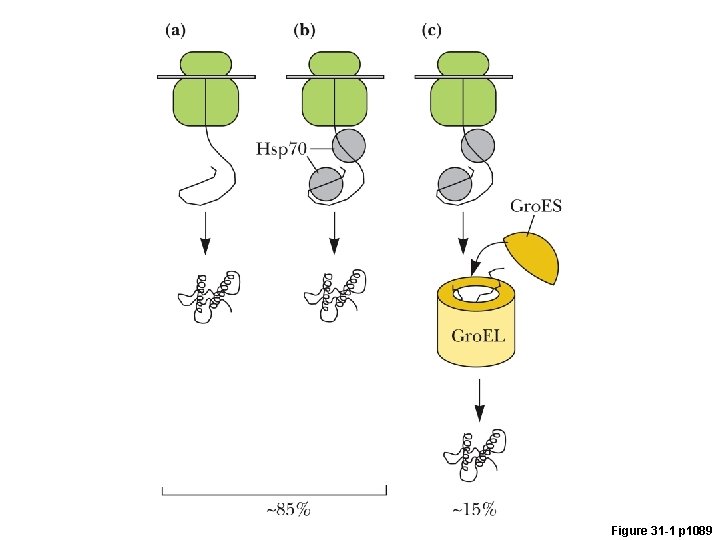
Figure 31 -1 p 1089
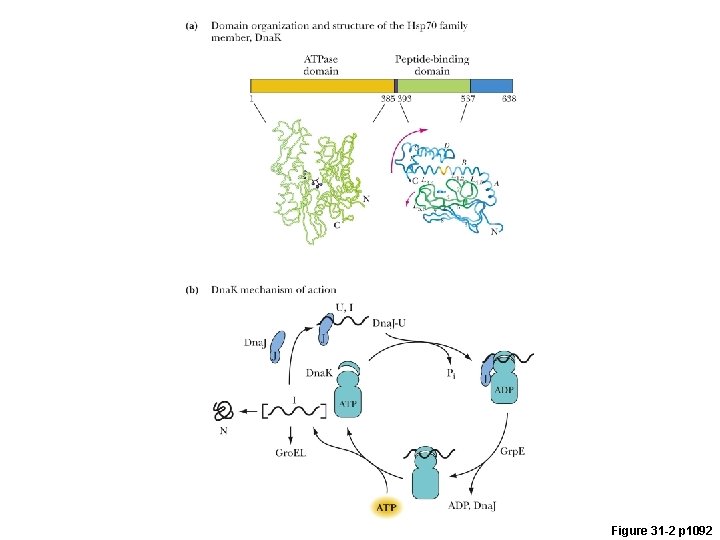
Figure 31 -2 p 1092
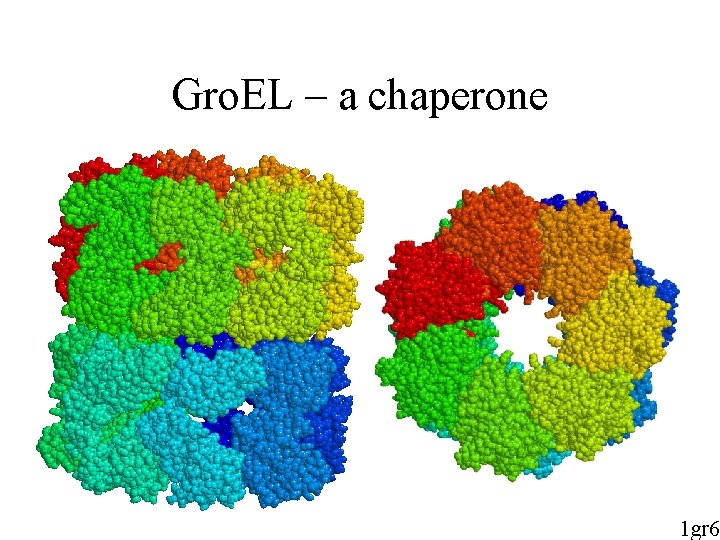
Gro. EL – a chaperone 1 gr 6
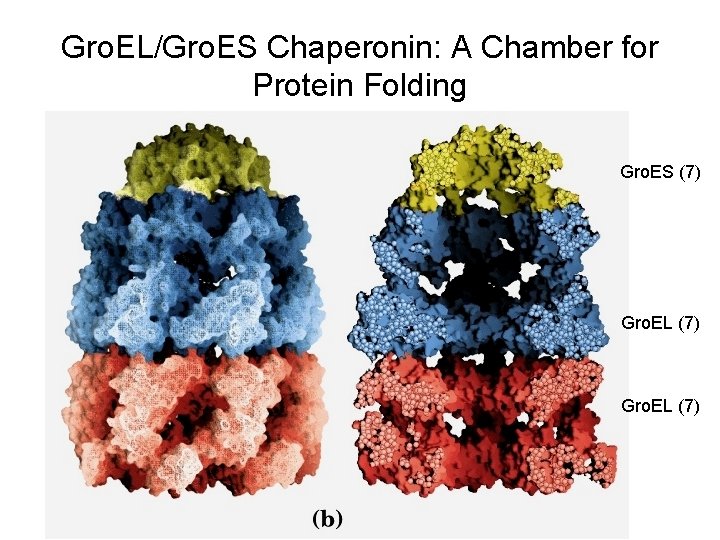
Gro. EL/Gro. ES Chaperonin: A Chamber for Protein Folding Gro. ES (7) Gro. EL (7)
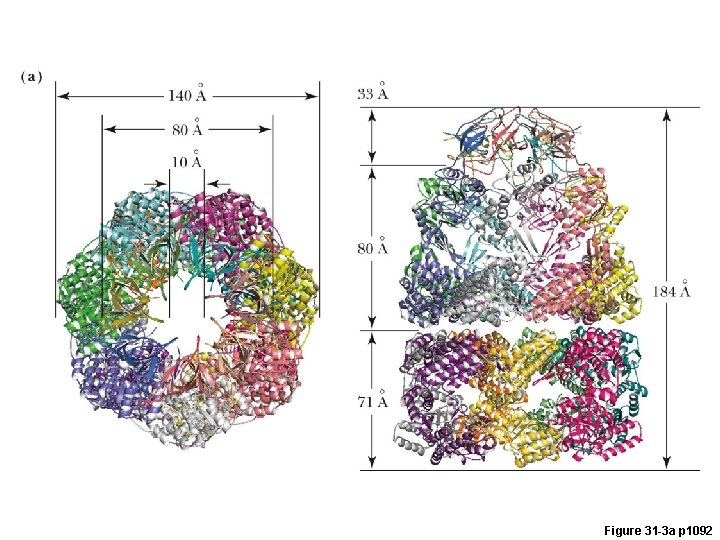
Figure 31 -3 a p 1092
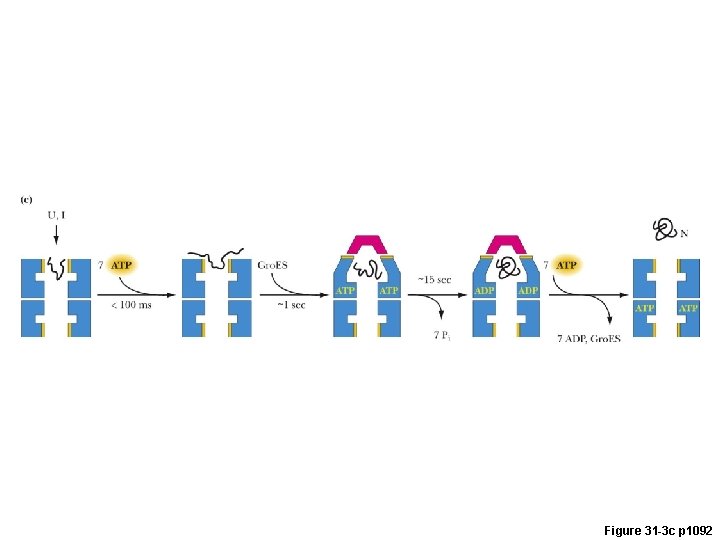
Figure 31 -3 c p 1092
Figure 31 -3 d p 1092
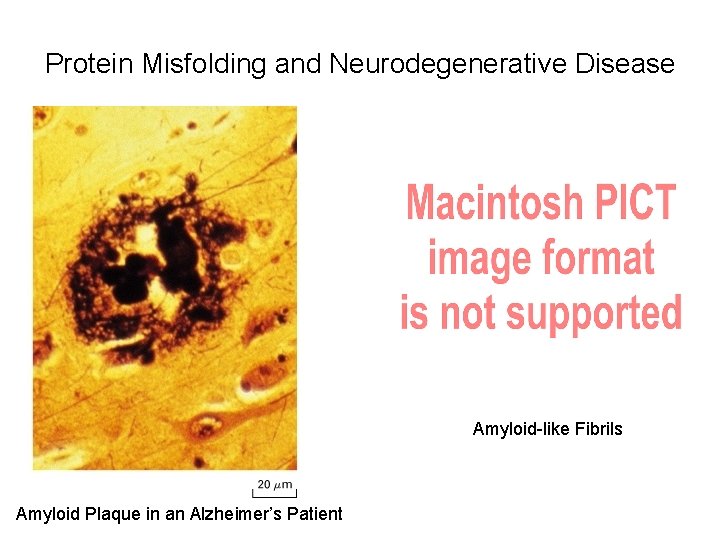
Protein Misfolding and Neurodegenerative Disease Amyloid-like Fibrils Amyloid Plaque in an Alzheimer’s Patient
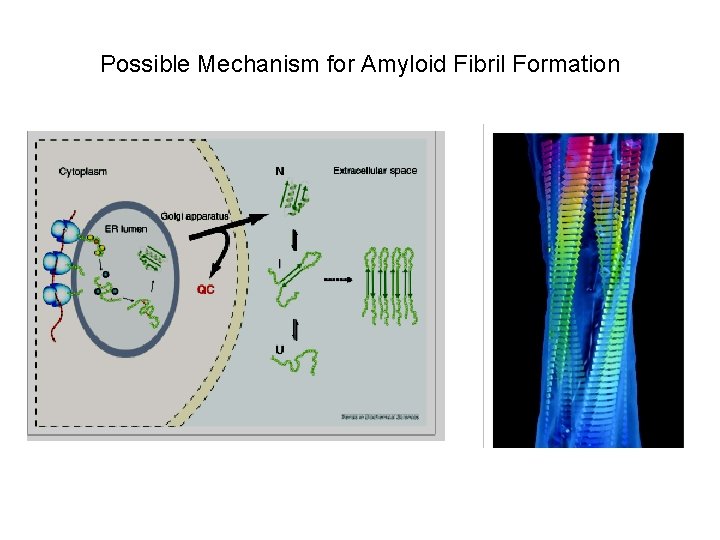
Possible Mechanism for Amyloid Fibril Formation
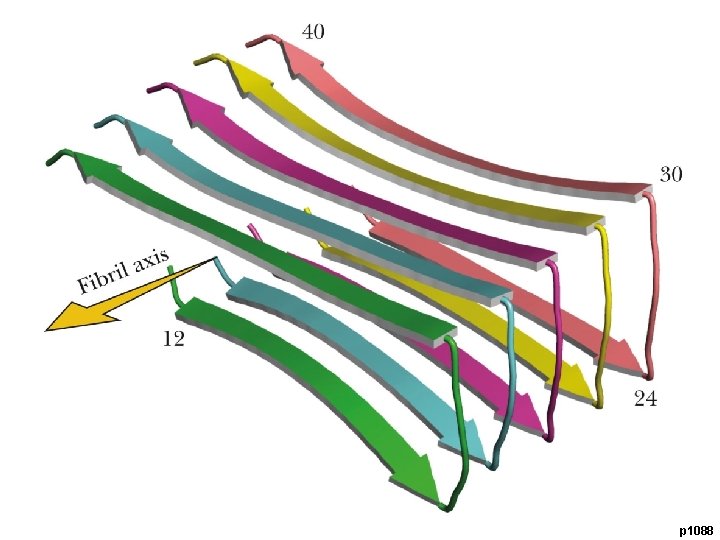
p 1088
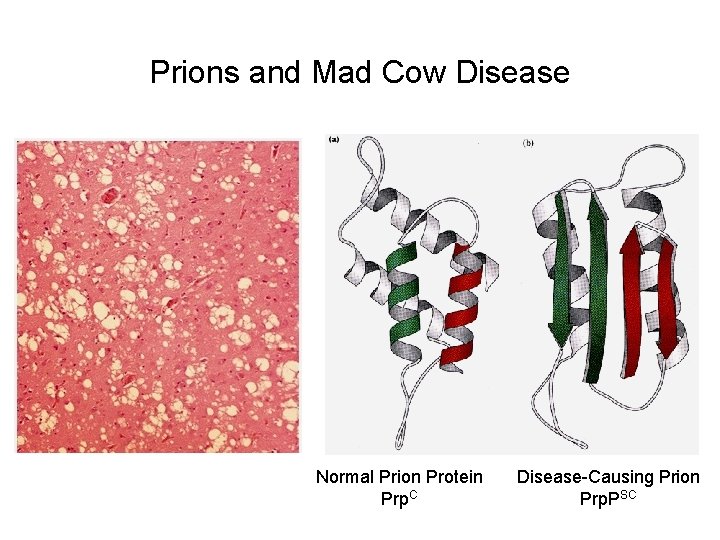
Prions and Mad Cow Disease Normal Prion Protein Prp. C Disease-Causing Prion Prp. PSC
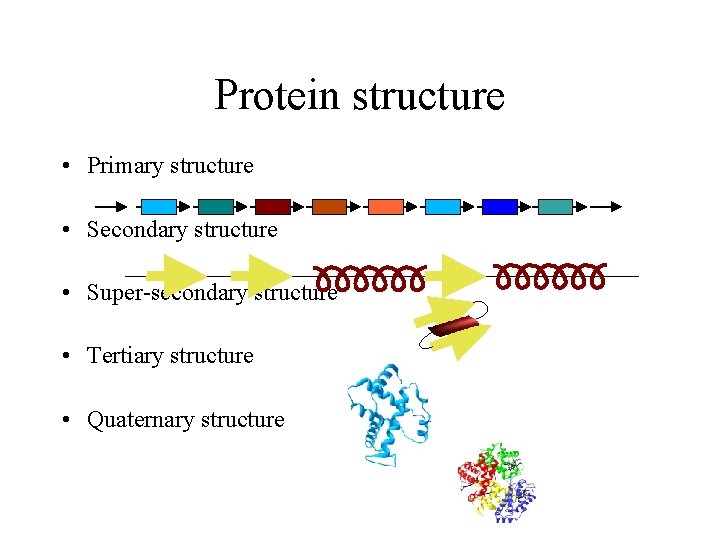
Protein structure • Primary structure • Secondary structure • Super-secondary structure • Tertiary structure • Quaternary structure
- Slides: 21