Biochemical Cycles in the Ecosystem Review How does
Biochemical Cycles in the Ecosystem Review: How does air pollution affect the carbon cycle
Some biogeochemical cycling key points: • cycling occurs at local to global scales • biogeochemical cycles have 2 basic parts: pools and fluxes • elements are recycled among the biosphere, atmosphere, lithosphere and hydrosphere • cycles of each element differ (chemistry, rates, pools, fluxes, interactions) • cycling is important because it can affect many other aspects of the environment and the quality of our lives
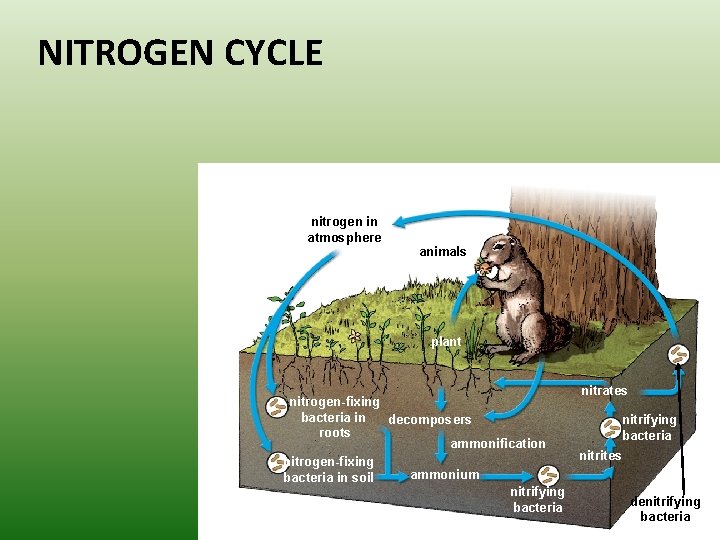
NITROGEN CYCLE nitrogen in atmosphere animals plant nitrogen-fixing bacteria in decomposers roots ammonification nitrogen-fixing ammonium bacteria in soil nitrifying bacteria nitrates nitrifying bacteria nitrites denitrifying bacteria

The nitrogen cycle mostly takes place underground – Some bacteria convert gaseous nitrogen into ammonia through a process called nitrogen fixation. – Some nitrogen-fixing bacteria live in nodules on the roots of plants; others live freely in the soil.

Ammonia released into the soil is transformed into ammonium. –Nitrifying bacteria change the ammonium into nitrate. –Nitrogen moves through the food web and returns to the soil during decomposition.
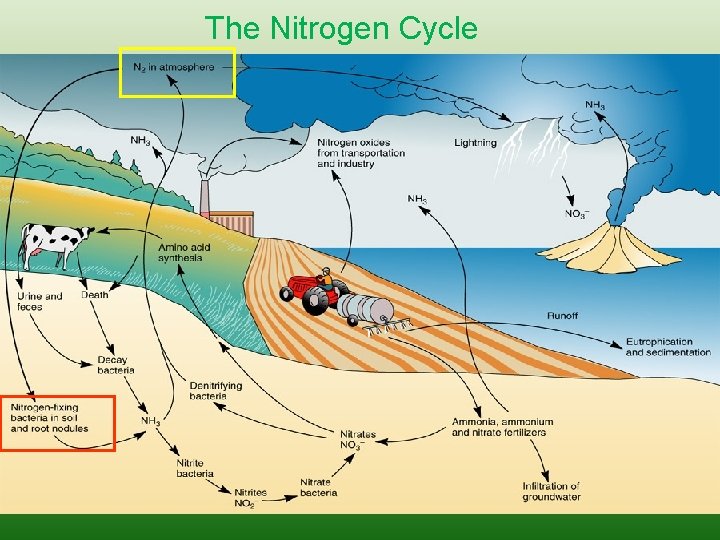
The Nitrogen Cycle

Nitrogen Fixation The nodules on the roots of this bean plant contain bacteria called Rhizobium that help convert nitrogen in the soil to a form the plant can utilize.

Nitrogen Cycling Processes Nitrogen Fixation – bacteria convert nitrogen gas (N 2) to ammonia (NH 3). Decomposition – dead nitrogen fixers release Ncontaining compounds. Ammonification – bacteria and fungi decompose dead plants and animals and release excess NH 3 and ammonium ions (NH 4+). Nitrification – type of chemosynthesis where NH 3 or NH 4+ is converted to nitrite (NO 2 -); other bacteria convert NO 2 - to nitrate (NO 3 -). Denitrification – bacteria convert NO 2 - and NO 3 - to N 2.

Sources • • • Lightning Inorganic fertilizers Nitrogen Fixation Animal Residues Crop residues Organic fertilizers

Forms of Nitrogen • • Urea CO(NH 2)2 Ammonia NH 3 (gaseous) Ammonium NH 4 Nitrate NO 3 Nitrite NO 2 Atmospheric Dinitrogen N 2 Organic N
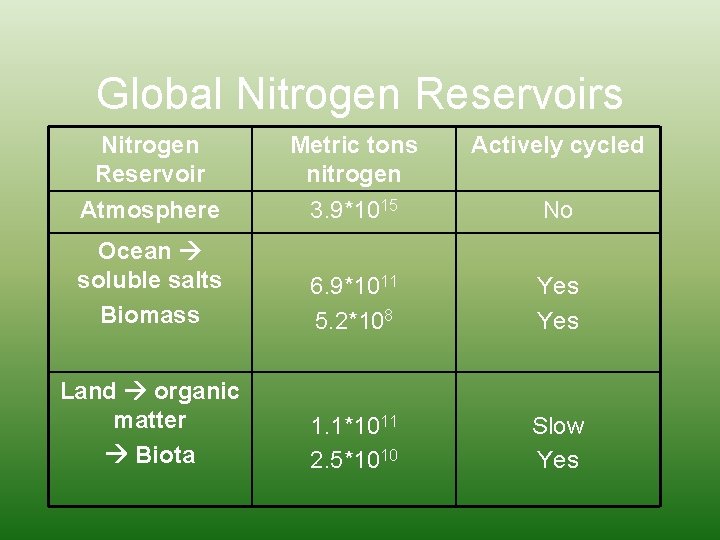
Global Nitrogen Reservoirs Nitrogen Reservoir Metric tons nitrogen Actively cycled Atmosphere 3. 9*1015 No Ocean soluble salts Biomass 6. 9*1011 5. 2*108 Yes Land organic matter Biota 1. 1*1011 2. 5*1010 Slow Yes

Roles of Nitrogen • Plants and bacteria use nitrogen in the form of NH 4+ or NO 3 • It serves as an electron acceptor in anaerobic environment • Nitrogen is often the most limiting nutrient in soil and water.

Nitrogen is a key element for • amino acids • nucleic acids (purine, pyrimidine) • cell wall components of bacteria (NAM).
Humans Impacts on Nitrogen Cycle • Burning fossil fuels, • application of nitrogen-based fertilizers

Human additions to Nitrogen Cycle • • • nutrient imbalance in trees, changes in forest health declines in biodiversity make its way into our drinking water. can lead to increased acidification in freshwater ecosystems lead to anoxia (no oxygen) or hypoxia (low oxygen) in aquatic systems changes in food-web structure increase in harmful algal blooms may lead to an increased risk of parasitic and infectious diseases among humans and wildlife
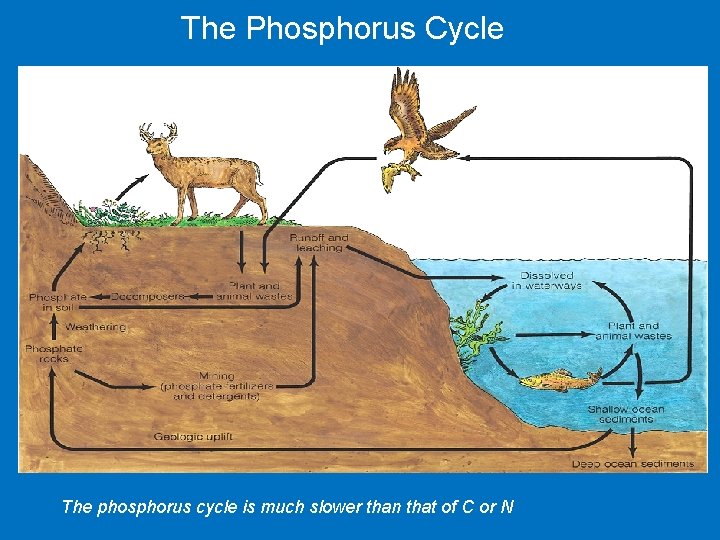
The Phosphorus Cycle The phosphorus cycle is much slower than that of C or N

The phosphorus cycle takes place at and below ground level – Phosphate is released by the weathering of rocks. – Phosphorus moves through the food web and returns to the soil during decomposition. – Phosphorus leaches into groundwater from the soil and is locked in sediments. – Both mining and agriculture add phosphorus into the environment.

Phosphorus • Extremely local recycling (no gaseous phase) • Long-term weathering/erosion cycle • Most important/limiting in aquatic ecosystems, tropical terrestrial habitats

HUMAN IMPACTS TO PHOSPHOROUS CYCLE 1. Humans mine LARGE quantities of phosphate rock to use in commercial fertilizers and detergents. Phosphorous is NOT found as a gas, only as a solid in the earth’s crust. It takes millions to hundreds of millions of years to replenish. 2. Phosphorous is held in the tissue of the trees and vegetation, not in the soil and as we deforest the land, we remove the ability for phosphorous to replenish globally in ecosystems. 3. Cultural eutrophication – ad excess phosphate to aquatic ecosystems in runoff of animal wastes from livestock feedlots, runoff of commercial phosphate fertilizers fro cropland, and discharge of municipal sewage.
IMPORTANCE OF PHOSPHOROUS CYCLE • Phosphorous is an essential nutrient of both plants and animals. • It is part of DNA molecules which carry genetic information. • It is part of ATP and ADP) that store chemical energy for use by organisms in cellular respiration. • Forms phospholipids in cell membranes of plants and animal cells. • Forms bones, teeth, and shells of animals as calcium phosphate compounds.
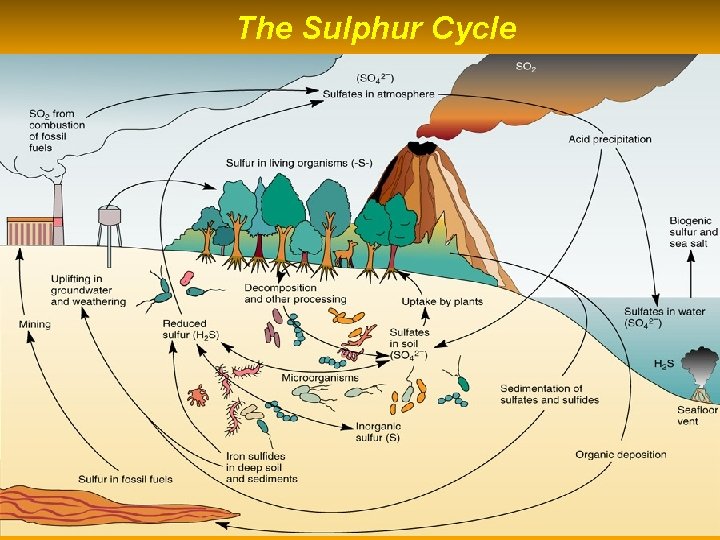
The Sulphur Cycle
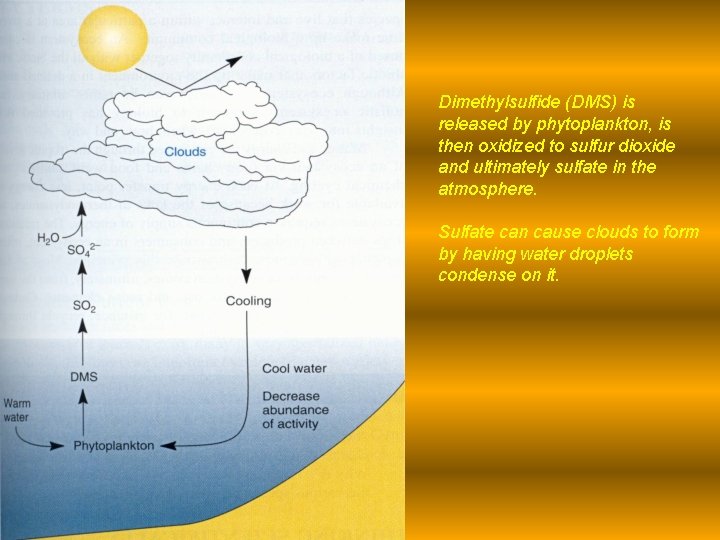
Dimethylsulfide (DMS) is released by phytoplankton, is then oxidized to sulfur dioxide and ultimately sulfate in the atmosphere. Sulfate can cause clouds to form by having water droplets condense on it.

HUMAN IMPACTS TO SULFUR CYCLE Approximately 1/3 of all sulfur emitted into atmosphere comes from human activities. • 1. Burning sulfur containing coal and oil to produce electric power (SOx = acid deposition). • 2. Refining petroleum – (SOx emissions) • 3. Smelting to convert sulfur compounds of metallic minerals into free metals (Cu, Pb, Zn) • 4. Industrial processing.

IMPORTANCE OF SULFUR CYCLE 1. Sulfur is a component of most proteins and some vitamins. 2. Sulfate ions (SO 4 2 - ) dissolved in water are common in plant tissue. They are part of sulfur-containing amino acids that are the building blocks for proteins. 3. Sulfur bonds give three dimensional structure of amino acids. 4. Many animals, including humans, depend on plants for sulfur-containing amino acids.
Homework Draw a picture showing how these cycles interact with the Biosphere

Application Human populations are increasing at a staggering rate. How does this increase affect the cycling of carbon, nitrogen, and phosphorous? Does the excess or depletion of these minerals change the diversity of an ecosystem? What are some solutions to these problems?

Closing Without these minerals cycling the ecosystem would collapse
- Slides: 27