Bioavailability Distribution Absorption and Transport Volume of Distribution

Bioavailability Distribution Absorption and Transport

Volume of Distribution The volume of distribution is given by the following equation: Therefore the dose required to give a certain plasma concentration can be determined if the VD for that drug is known. The VD is not a physiologic value; it is more a reflection of how a drug will distribute throughout the body depending on several physicochemical properties, e. g. solubility, charge, size, etc. VD is often calculated per weight of body – it is not volume any more!
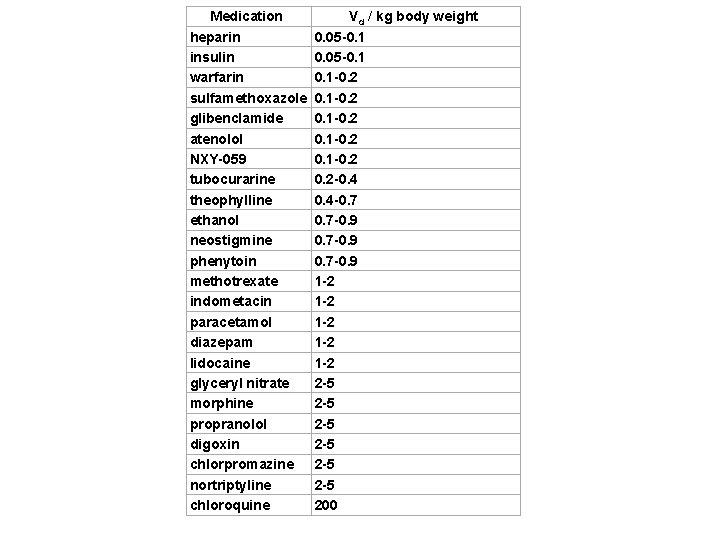
Medication heparin insulin warfarin sulfamethoxazole glibenclamide atenolol NXY-059 tubocurarine theophylline ethanol neostigmine phenytoin methotrexate indometacin paracetamol diazepam lidocaine glyceryl nitrate morphine propranolol digoxin chlorpromazine nortriptyline chloroquine Vd / kg body weight 0. 05 -0. 1 -0. 2 0. 1 -0. 2 -0. 4 -0. 7 -0. 9 0. 7 -0. 9 1 -2 1 -2 1 -2 2 -5 2 -5 2 -5 200
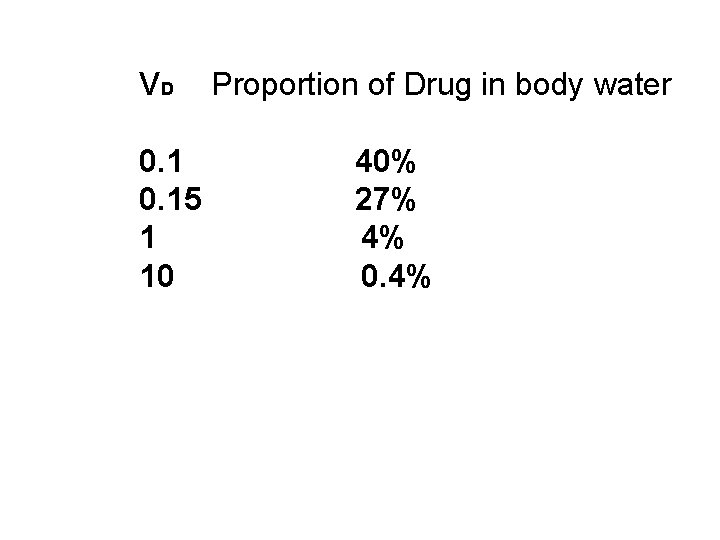
VD 0. 15 1 10 Proportion of Drug in body water 40% 27% 4% 0. 4%
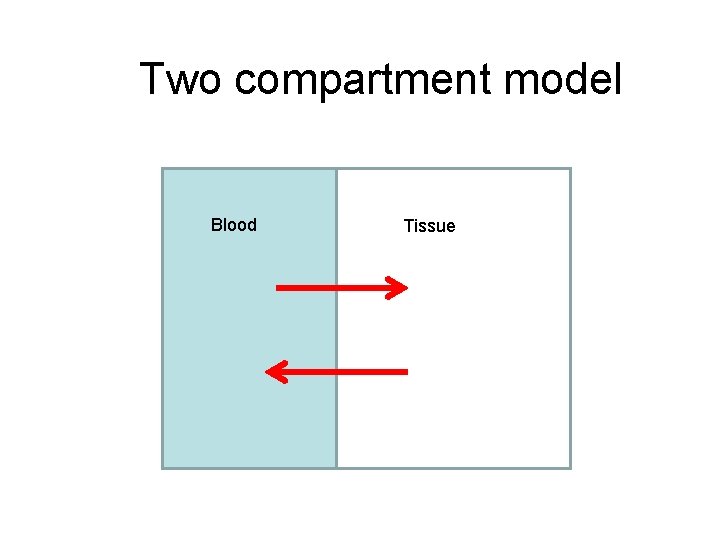
Two compartment model Blood Tissue
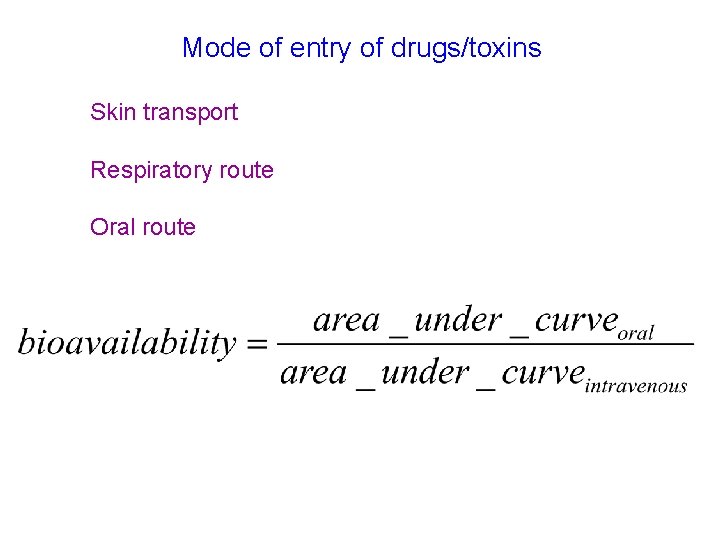
Mode of entry of drugs/toxins Skin transport Respiratory route Oral route
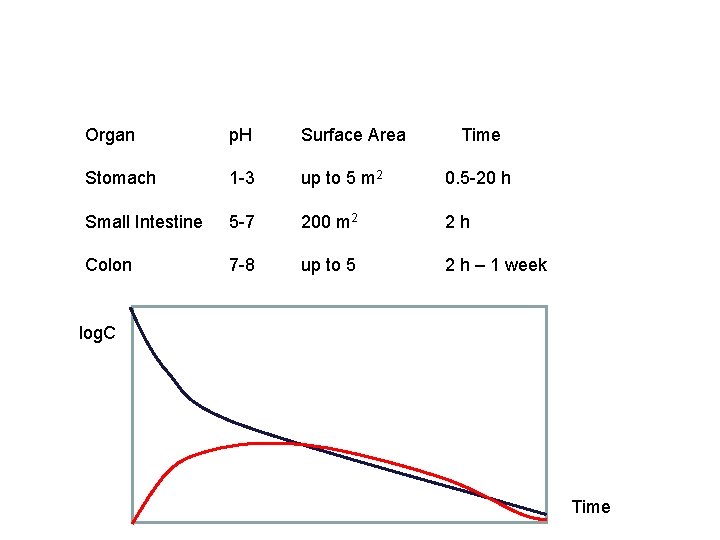
Organ p. H Surface Area Time Stomach 1 -3 up to 5 m 2 0. 5 -20 h Small Intestine 5 -7 200 m 2 2 h Colon 7 -8 up to 5 2 h – 1 week log. C Time
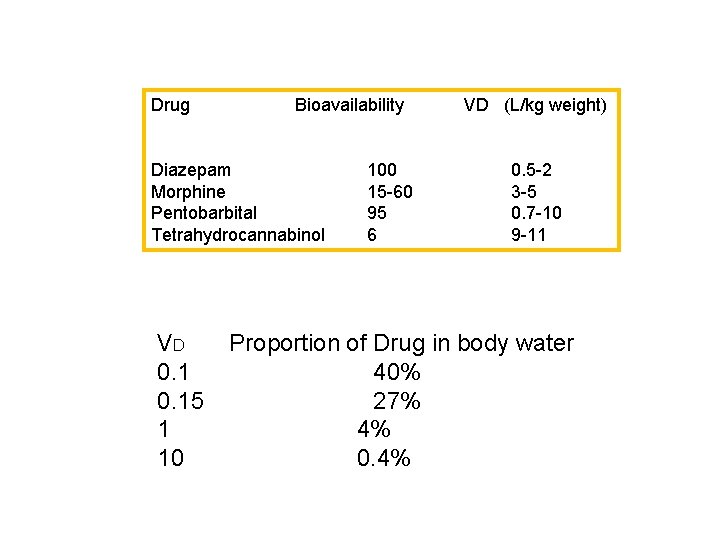
Drug Bioavailability Diazepam Morphine Pentobarbital Tetrahydrocannabinol VD 0. 15 1 10 100 15 -60 95 6 VD (L/kg weight) 0. 5 -2 3 -5 0. 7 -10 9 -11 Proportion of Drug in body water 40% 27% 4% 0. 4%
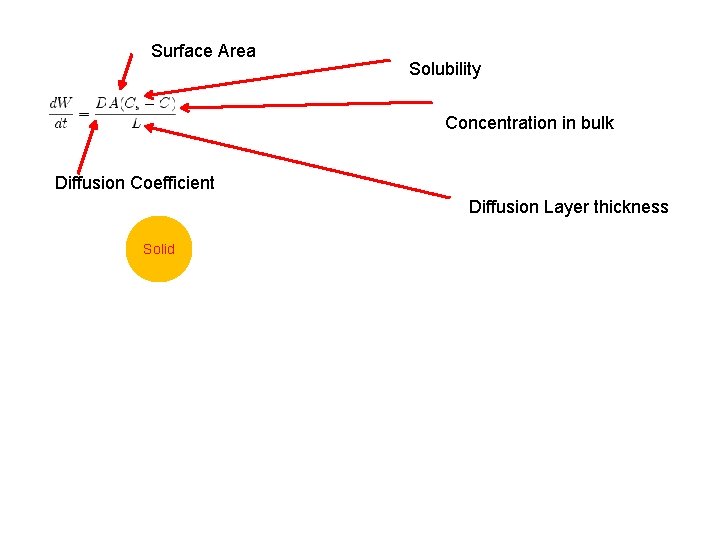
Surface Area Solubility Concentration in bulk Diffusion Coefficient Diffusion Layer thickness Solid
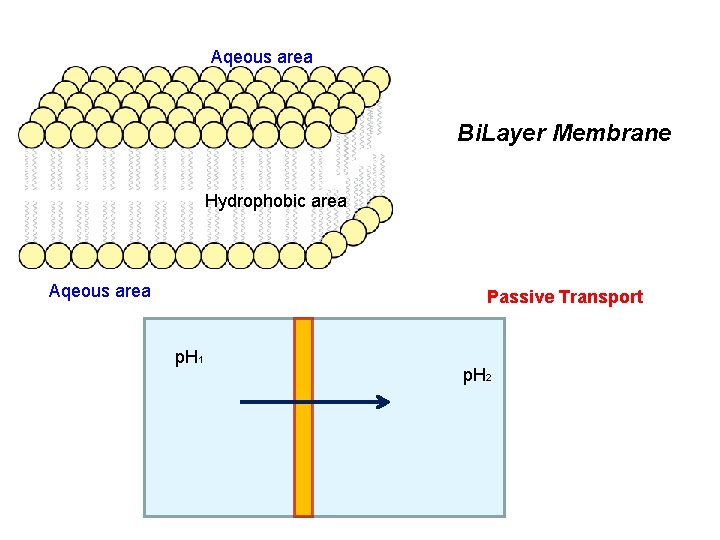
Aqeous area Bi. Layer Membrane Hydrophobic area Aqeous area Passive Transport p. H 1 p. H 2
- Slides: 10