Bioabsorbable Polymer DES SYNERGY Update Dean J Kereiakes

Bioabsorbable Polymer DES: SYNERGY Update Dean J. Kereiakes, M. D. Medical Director, The Christ Hospital Heart and Vascular Center and the Lindner Research Center, Cincinnati, Ohio Professor of Medicine, Ohio State University

Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship • • Modest Consulting Fees Significant Consulting Fees Significant Consulting Fees Major Stock Shareholder/Equity Company • • HCRI Boston Scientific Abbott Vascular Svelte Medical Systems, Inc. Janssen Research & Development LLC Sanofi-Aventis U. S. LLC Ablative Solutions, Inc.
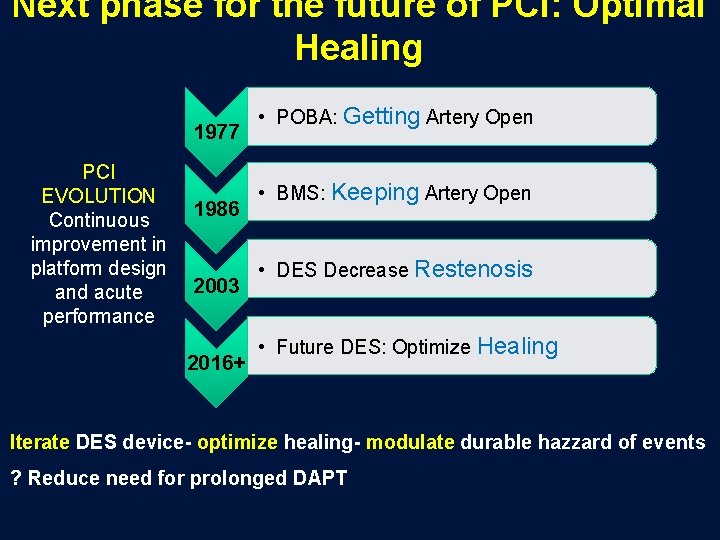
Next phase for the future of PCI: Optimal Healing 1977 PCI EVOLUTION Continuous improvement in platform design and acute performance 1986 2003 2016+ • POBA: Getting Artery Open • BMS: Keeping Artery Open • DES Decrease Restenosis • Future DES: Optimize Healing Iterate DES device- optimize healing- modulate durable hazzard of events ? Reduce need for prolonged DAPT
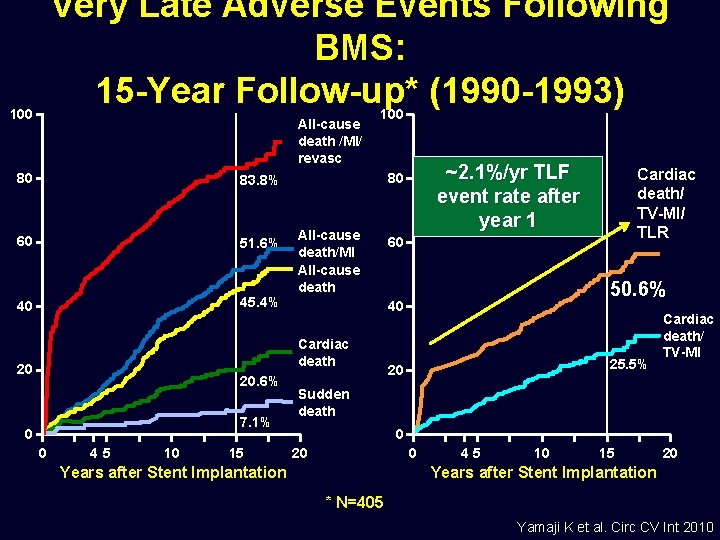
Very Late Adverse Events Following BMS: 15 -Year Follow-up* (1990 -1993) 100 All-cause death /MI/ revasc 80 83. 8% 60 51. 6% 40 45. 4% 100 All-cause death/MI All-cause death 20. 6% 7. 1% 0 0 45 10 15 Cardiac death/ TV-MI/ TLR 60 50. 6% 40 Cardiac death 20 ~2. 1%/yr TLF event rate after year 1 80 25. 5% 20 Cardiac death/ TV-MI Sudden death 0 20 0 Years after Stent Implantation 45 10 15 20 Years after Stent Implantation * N=405 Yamaji K et al. Circ CV Int 2010
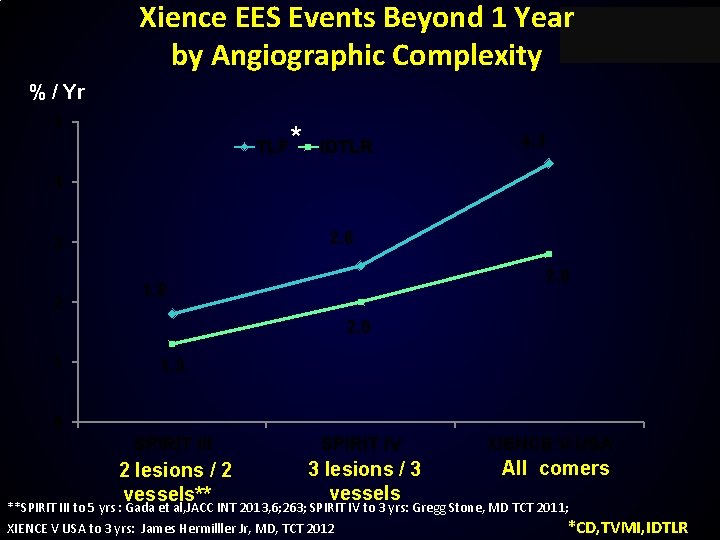
Xience EES Events Beyond 1 Year by Angiographic Complexity % / Yr 5 TLF * IDTLR 4. 3 4 2. 6 3 2 2. 8 1. 8 2. 0 1 1. 3 0 SPIRIT III SPIRIT IV 2 lesions / 2 vessels** 3 lesions / 3 vessels XIENCE V USA All comers **SPIRIT III to 5 yrs : Gada et al, JACC INT 2013, 6; 263; SPIRIT IV to 3 yrs: Gregg Stone, MD TCT 2011; XIENCE V USA to 3 yrs: James Hermilller Jr, MD, TCT 2012 *CD, TVMI, IDTLR

SYNERGY Stent Design Iterations: Everolimus Drug PLGA Polymer Platform Platinum chromium • 74 μm (0. 0029 in) • Radial Strength, >visibility • Recoil • Flexibility, conformability Drug & Polymer Coating Abluminal (4μm) SEM of coating (x 5000) Luminal Polymer Coating PLGA • Abluminal • 4 µm thick • 85: 15 ratio Drug Everolimus • 100 μg/cm 2
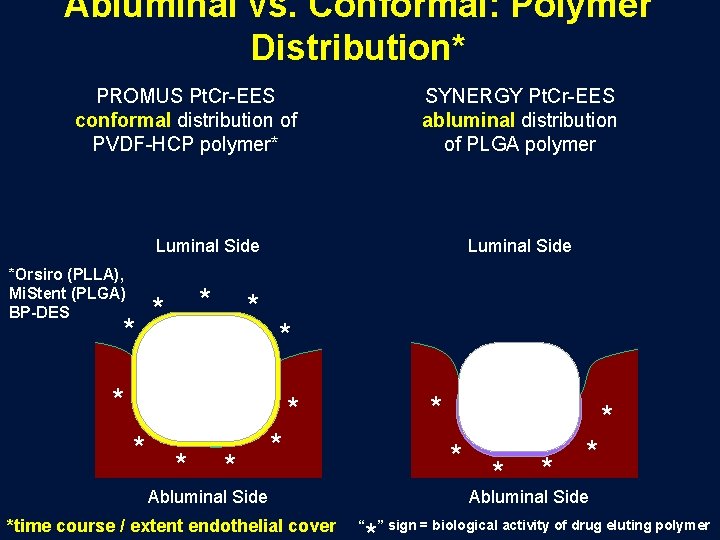
Abluminal vs. Conformal: Polymer Distribution* PROMUS Pt. Cr-EES conformal distribution of PVDF-HCP polymer* SYNERGY Pt. Cr-EES abluminal distribution of PLGA polymer Luminal Side *Orsiro (PLLA), Mi. Stent (PLGA) BP-DES * * Luminal Side * * * * Abluminal Side *time course / extent endothelial cover * * * Abluminal Side “ ” sign = biological activity of drug eluting polymer

Abluminal vs. Conformal Polymer Abluminal coating significantly improves endothelialization % Endothelial Cell (EC) Coverage at 21 Days in Cell Assay P<0. 001 * * * Conformal * * * * Abluminal only From data presented by Mike Eppihimer, Ph. D at Euro. PCR 2013
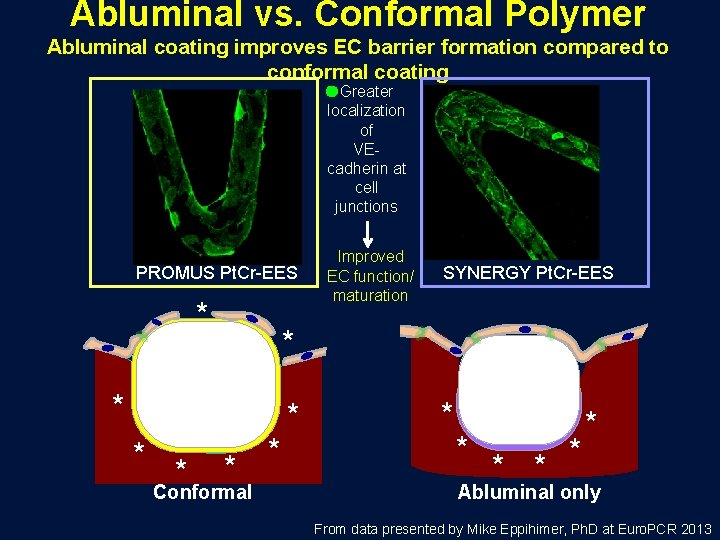
Abluminal vs. Conformal Polymer Abluminal coating improves EC barrier formation compared to conformal coating Greater localization of VEcadherin at cell junctions PROMUS Pt. Cr-EES * * SYNERGY Pt. Cr-EES * * * Improved EC function/ maturation * Conformal * * * * Abluminal only From data presented by Mike Eppihimer, Ph. D at Euro. PCR 2013
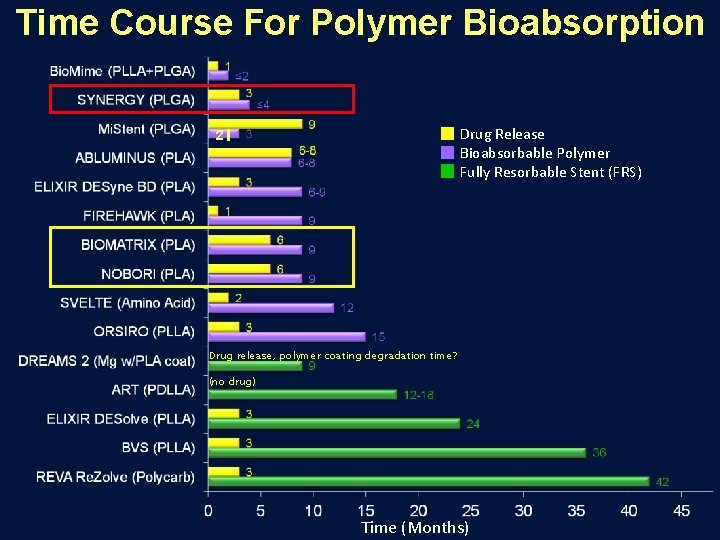
Time Course For Polymer Bioabsorption Drug Release Bioabsorbable Polymer Fully Resorbable Stent (FRS) 2 Drug release, polymer coating degradation time? (no drug) Time (Months)
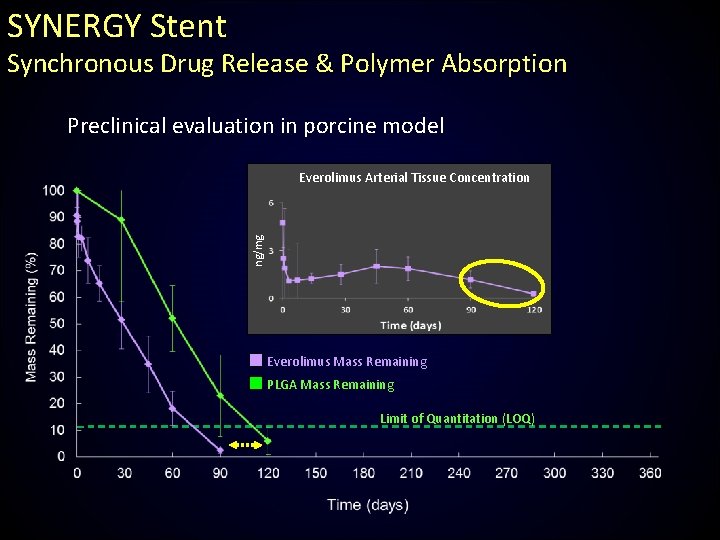
SYNERGY Stent Synchronous Drug Release & Polymer Absorption Preclinical evaluation in porcine model ng/mg Everolimus Arterial Tissue Concentration Everolimus Mass Remaining PLGA Mass Remaining Limit of Quantitation (LOQ)
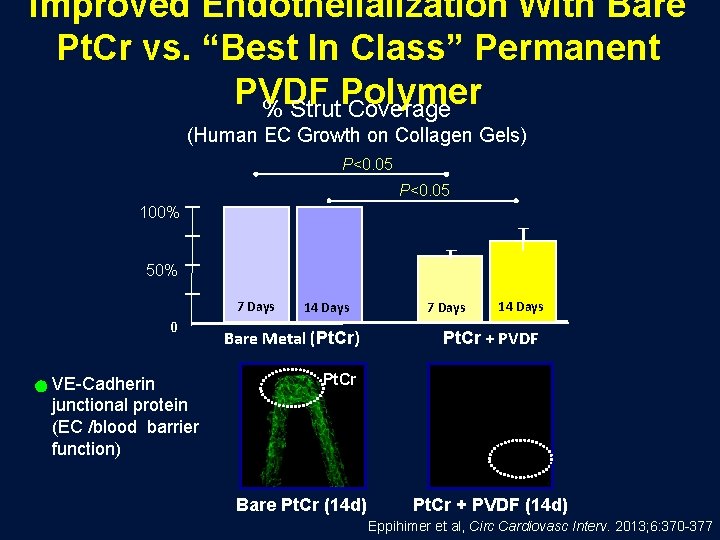
Improved Endothelialization With Bare Pt. Cr vs. “Best In Class” Permanent PVDF Polymer % Strut Coverage (Human EC Growth on Collagen Gels) P<0. 05 100% 50% 7 Days 0 VE-Cadherin junctional protein (EC /blood barrier function) 14 Days Bare Metal (Pt. Cr) 7 Days 14 Days Pt. Cr + PVDF Pt. Cr Bare Pt. Cr (14 d) Pt. Cr + PVDF (14 d) Eppihimer et al, Circ Cardiovasc Interv. 2013; 6: 370 -377
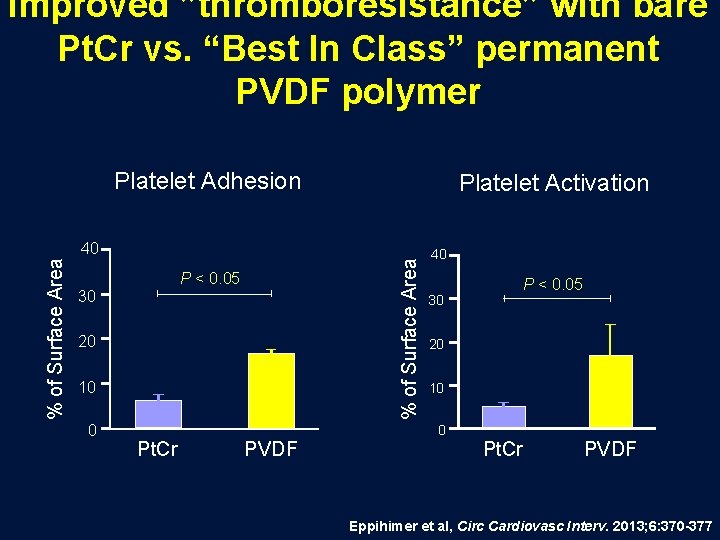
Improved ”thromboresistance” with bare Pt. Cr vs. “Best In Class” permanent PVDF polymer Platelet Adhesion Platelet Activation % of Surface Area 40 P < 0. 05 30 20 10 0 40 30 20 10 0 Pt. Cr PVDF P < 0. 05 Pt. Cr PVDF Eppihimer et al, Circ Cardiovasc Interv. 2013; 6: 370 -377
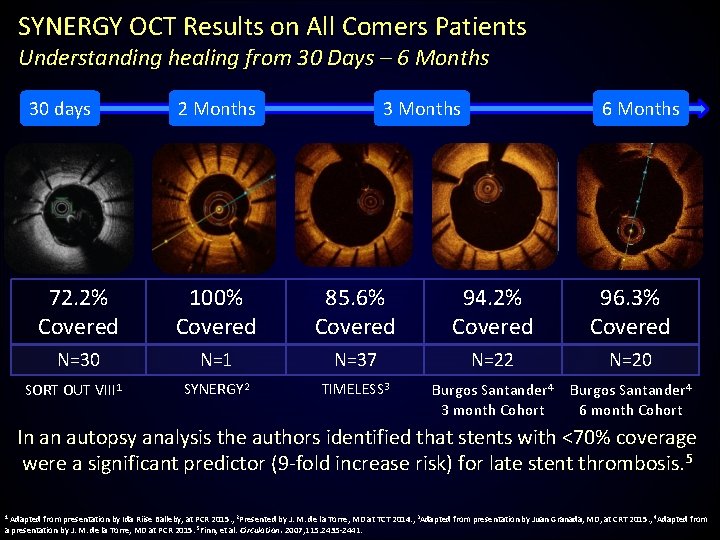
SYNERGY OCT Results on All Comers Patients Understanding healing from 30 Days – 6 Months 30 days 2 Months 3 Months 6 Months 72. 2% Covered 100% Covered 85. 6% Covered 94. 2% Covered 96. 3% Covered N=30 N=1 N=37 N=22 N=20 SYNERGY 2 TIMELESS 3 Burgos Santander 4 3 month Cohort Burgos Santander 4 6 month Cohort SORT OUT VIII 1 In an autopsy analysis the authors identified that stents with <70% coverage were a significant predictor (9 -fold increase risk) for late stent thrombosis. 5 1 Adapted from presentation by Ida Riise Balleby, at PCR 2015. , 2 Presented by J. M. de la Torre, MD at TCT 2014. , 3 Adapted from presentation by Juan Granada, MD, at CRT 2015. , 4 Adapted from a presentation by J. M. de la Torre, MD at PCR 2015. 5 Finn, et al. Circulation. 2007; 115: 2435 -2441.
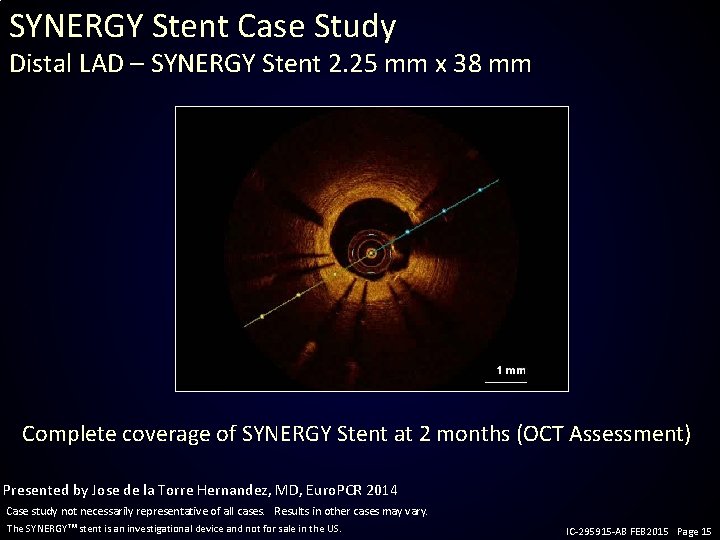
SYNERGY Stent Case Study Distal LAD – SYNERGY Stent 2. 25 mm x 38 mm Complete coverage of SYNERGY Stent at 2 months (OCT Assessment) Presented by Jose de la Torre Hernandez, MD, Euro. PCR 2014 Case study not necessarily representative of all cases. Results in other cases may vary. The SYNERGY stent is an investigational device and not for sale in the US. IC-295915 -AB FEB 2015 Page 15
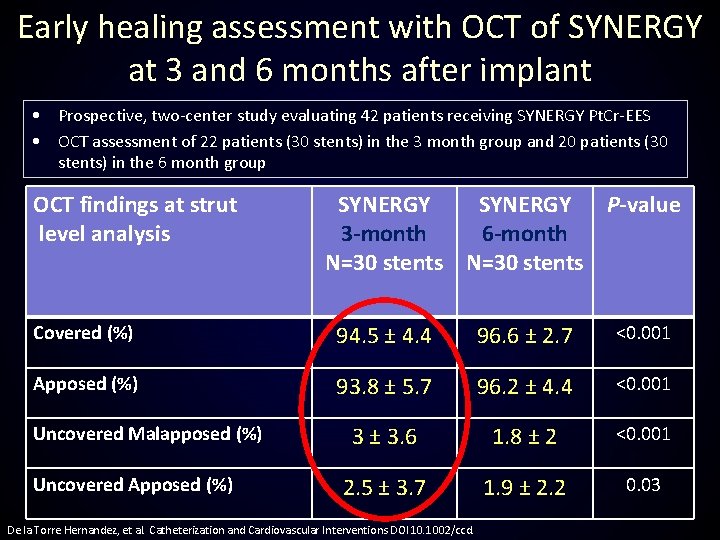
Early healing assessment with OCT of SYNERGY at 3 and 6 months after implant • Prospective, two-center study evaluating 42 patients receiving SYNERGY Pt. Cr-EES • OCT assessment of 22 patients (30 stents) in the 3 month group and 20 patients (30 stents) in the 6 month group OCT findings at strut level analysis SYNERGY P-value 3 -month 6 -month N=30 stents Covered (%) 94. 5 ± 4. 4 96. 6 ± 2. 7 <0. 001 Apposed (%) 93. 8 ± 5. 7 96. 2 ± 4. 4 <0. 001 3 ± 3. 6 1. 8 ± 2 <0. 001 2. 5 ± 3. 7 1. 9 ± 2. 2 0. 03 Uncovered Malapposed (%) Uncovered Apposed (%) De la Torre Hernandez, et al. Catheterization and Cardiovascular Interventions DOI 10. 1002/ccd.
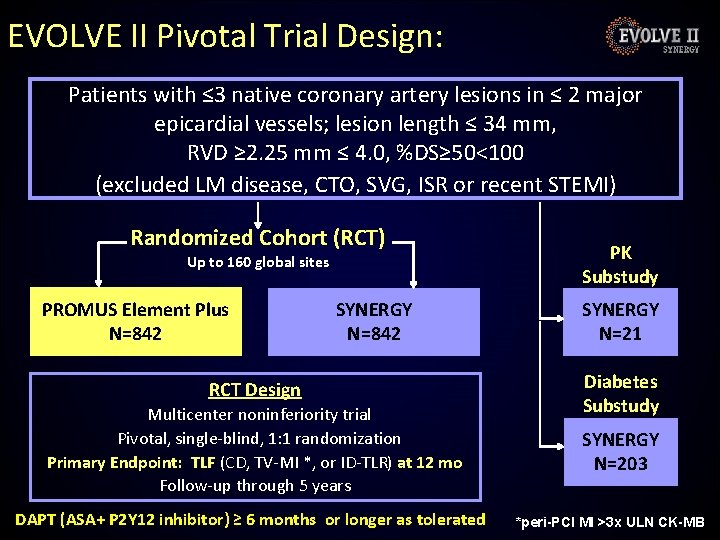
EVOLVE II Pivotal Trial Design: Patients with ≤ 3 native coronary artery lesions in ≤ 2 major epicardial vessels; lesion length ≤ 34 mm, RVD ≥ 2. 25 mm ≤ 4. 0, %DS≥ 50<100 (excluded LM disease, CTO, SVG, ISR or recent STEMI) Randomized Cohort (RCT) Up to 160 global sites PROMUS Element Plus N=842 SYNERGY N=842 RCT Design Multicenter noninferiority trial Pivotal, single-blind, 1: 1 randomization Primary Endpoint: TLF (CD, TV-MI *, or ID-TLR) at 12 mo Follow-up through 5 years DAPT (ASA+ P 2 Y 12 inhibitor) ≥ 6 months or longer as tolerated PK Substudy SYNERGY N=21 Diabetes Substudy SYNERGY N=203 *peri-PCI MI >3 x ULN CK-MB
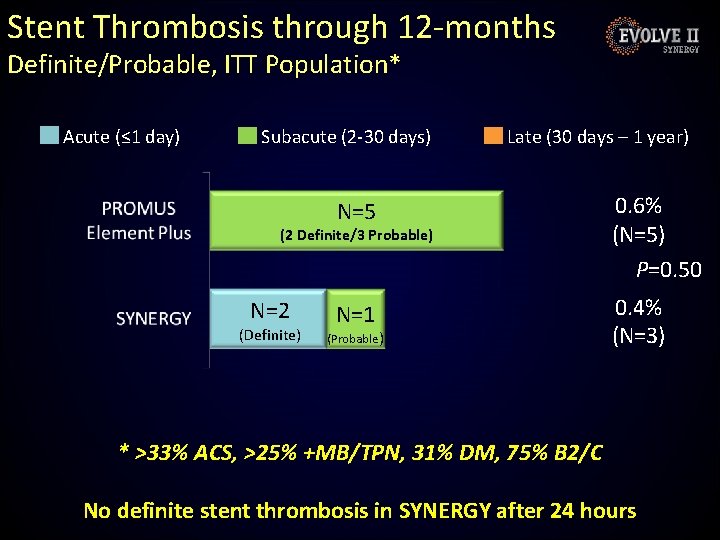
Stent Thrombosis through 12 -months Definite/Probable, ITT Population* Acute (≤ 1 day) Subacute (2 -30 days) Late (30 days – 1 year) N=5 0. 6% (N=5) P=0. 50 N=1 0. 4% (N=3) (2 Definite/3 Probable) N=2 (Definite) (Probable) * >33% ACS, >25% +MB/TPN, 31% DM, 75% B 2/C No definite stent thrombosis in SYNERGY after 24 hours
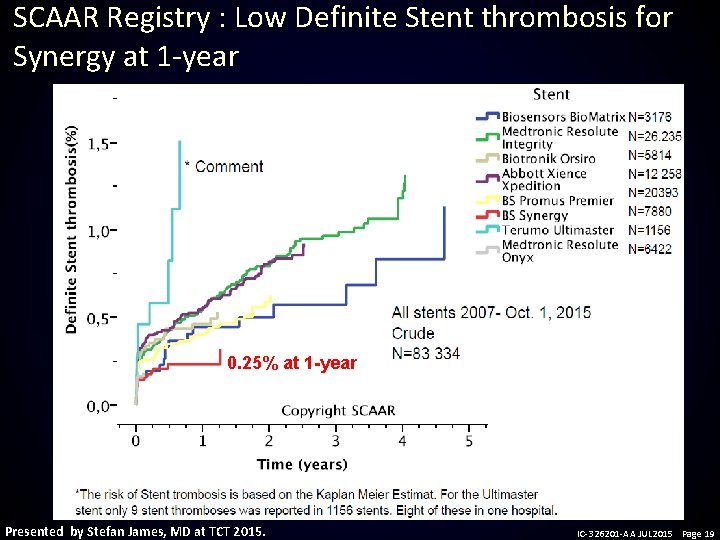
SCAAR Registry : Low Definite Stent thrombosis for Synergy at 1 -year 0. 25% at 1 -year Presented by Stefan James, MD at TCT 2015. IC-326201 -AA JUL 2015 Page 19
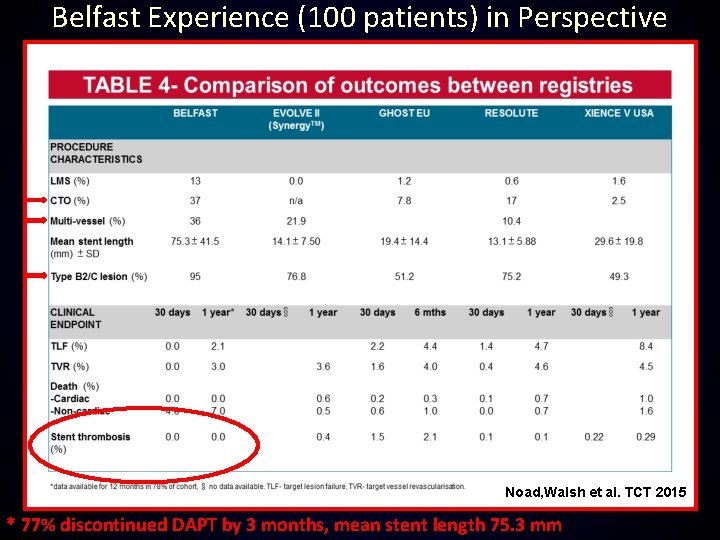
Belfast Experience (100 patients) in Perspective Noad, Walsh et al. TCT 2015 * 77% discontinued DAPT by 3 months, mean stent length 75. 3 mm
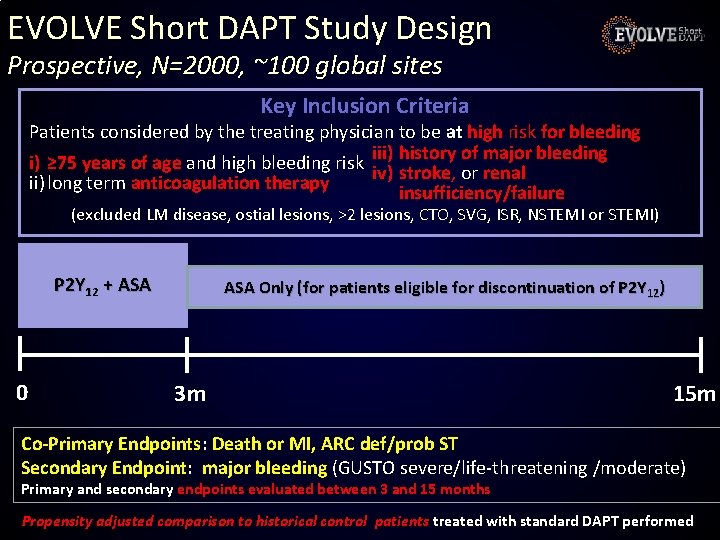
EVOLVE Short DAPT Study Design Prospective, N=2000, ~100 global sites Key Inclusion Criteria Patients considered by the treating physician to be at high risk for bleeding history of major bleeding i) ≥ 75 years of age and high bleeding risk iii) iv) stroke, or renal ii) long term anticoagulation therapy insufficiency/failure (excluded LM disease, ostial lesions, >2 lesions, CTO, SVG, ISR, NSTEMI or STEMI) P 2 Y 12 + ASA 0 ASA Only (for patients eligible for discontinuation of P 2 Y 12) 3 m 15 m Co-Primary Endpoints: Death or MI, ARC def/prob ST Secondary Endpoint: major bleeding (GUSTO severe/life-threatening /moderate) Primary and secondary endpoints evaluated between 3 and 15 months Propensity adjusted comparison to historical control patients treated with standard DAPT performed

SYNERGY : Conclusions and Significance § EVOLVE II pivotal non-inferiority trial proved that SYNERGY is noninferior to the Promus Element Plus stent for TLF at 1 year. § Despite clinical and angiographic complexity of study population, stent thrombosis rates were low. Definite ST not observed beyond 24 hrs following SYNERGY § Cummulative OCT experience with SYNERGY demonstrates expedited ( >90% within 3 months) stent healing § Accumulating “real world” clinical experience with SYNERGY suggests exceptional safety (very low ST) § A large scale clinical trial evaluating safety and efficacy of short (3 mos) DAPT in SYNERGY treated patients with increased bleeding risk is underway

Efficacy and Safety of a Novel Bioabsorbable Polymer-Coated, Everolimus-Eluting Coronary Stent: The EVOLVE II Randomized Trial Dean J. Kereiakes, MD; Ian T. Meredith, AM, MBBS, Ph. D; Stephan Windecker, MD; R. Lee Jobe, MD; Shamir R. Mehta, MD; Ian J. Sarembock, MBCh. B, MD; Robert L. Feldman, MD; Bernardo Stein, MD; Christophe Dubois, MD, Ph. D; Timothy Grady, DO; Shigeru Saito, MD; Takeshi Kimura, MD; Thomas Christen, MD, Ph. D; Dominic J. Allocco, MD; Keith D. Dawkins, MD Circ Card Intv 2015 Apr; 8(4). pii: e 002372 Doi: 10. 116/CIRCINTERVENTIONS. 114. 002372
- Slides: 23