BIO Life GEO CHEMICAL Earth Elements and molecules

BIO Life GEO CHEMICAL Earth Elements and molecules There are 3 essential biogeochemical cycles- the water cycle, the carbon cycle, and the nitrogen cycle. In order for these materials to be recycled, they must change states and transform!
Biogeochemical Cycles Recall that matter is neither created nor destroyed; but it can transform and be passed on. Biogeochemical cycles: how water, carbon, nitrogen and phosphorus pass from the physical environment to living organisms.
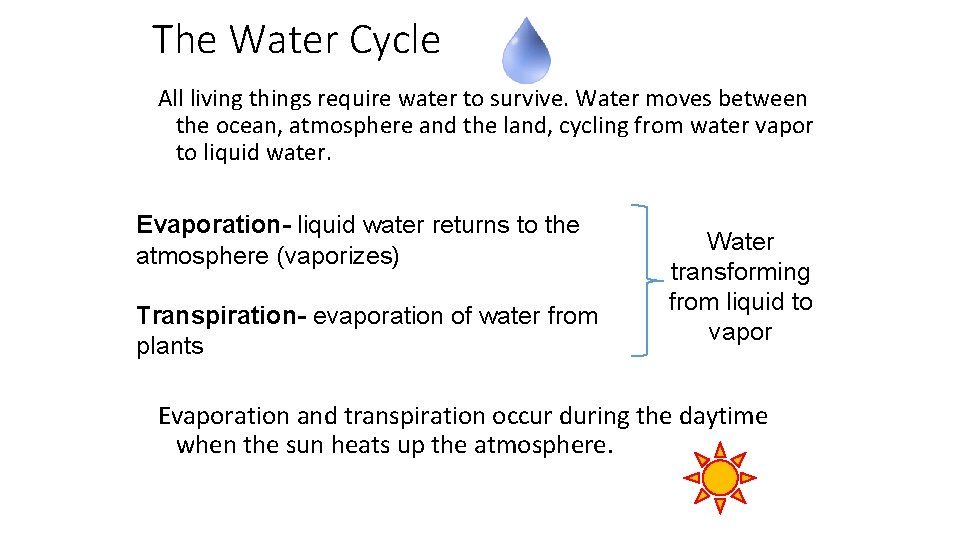
The Water Cycle All living things require water to survive. Water moves between the ocean, atmosphere and the land, cycling from water vapor to liquid water. Evaporation- liquid water returns to the atmosphere (vaporizes) Transpiration- evaporation of water from plants Water transforming from liquid to vapor Evaporation and transpiration occur during the daytime when the sun heats up the atmosphere.

Water’s Unique Properties • There are strong forces of attraction between molecules of water. • Water exists as a liquid over a wide temperature range. • Liquid water changes temperature slowly. • It takes a large amount of energy for water to evaporate. • Liquid water can dissolve a variety of compounds. • Water expands when it freezes.

As the atmosphere cools, the water vapor in the air condenses to form clouds, in a process called condensation. Precipitation- when the water droplets that form clouds become large enough, the water droplets fall to the earth (rain, sleet, snow). Once the water is returned to the earth, some of it is absorbed by plants through their roots. Other water continues to seep into the soil to become ground water in a process known as percolation. Runoff is surface water found on land that is eventually carried back to an ocean or lake. Water transforming from vapor to liquid
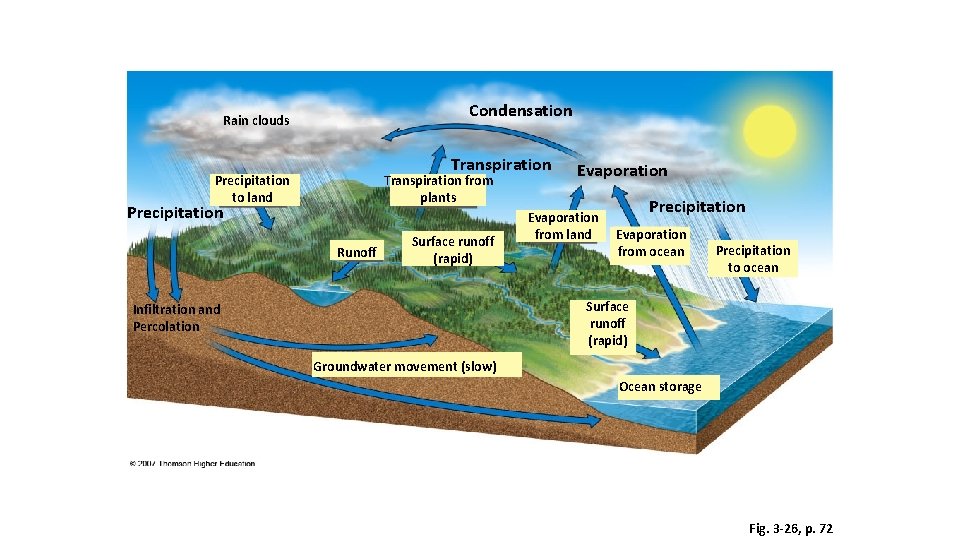
Condensation Rain clouds Transpiration Precipitation to land Transpiration from plants Precipitation Runoff Surface runoff (rapid) Evaporation from land Precipitation Evaporation from ocean Precipitation to ocean Surface runoff (rapid) Infiltration and Percolation Groundwater movement (slow) Ocean storage Fig. 3 -26, p. 72

Surface Run Off • Replenishes streams and lakes • Causes soil erosion • Sculpts the landscape • Transports nutrients
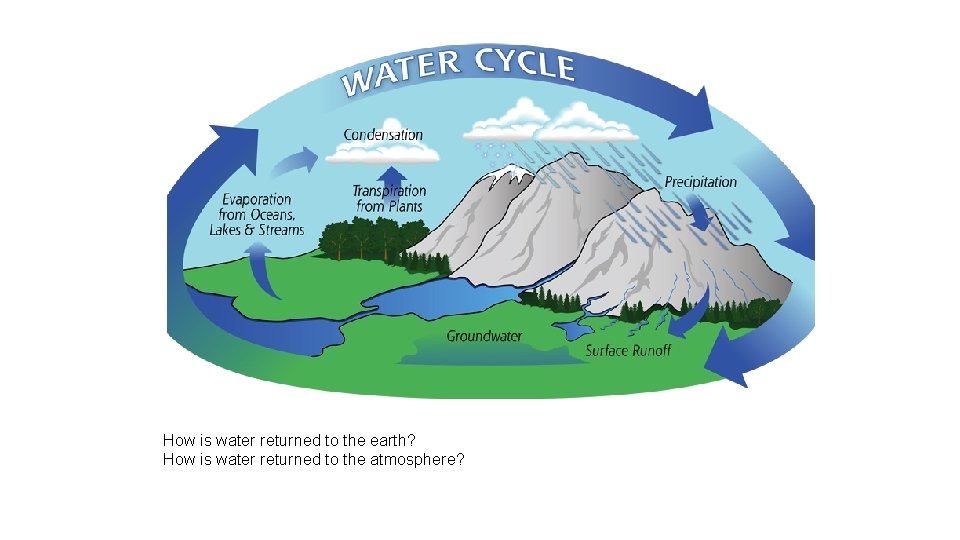
How is water returned to the earth? How is water returned to the atmosphere?

The Carbon Cycle Carbon is an essential element for all living things. Carbon is found in living tissues, rocks, the atmosphere, and the ocean. Less than 1% of the carbon found on earth participates in the carbon cycle. Carbon dioxide that is in the air or dissolved in water is used by photosynthesizing plants, algae and bacteria as a raw material to build organic molecules such as glucose.

The Carbon Cycle: Earth’s Thermostat • If the carbon cycle removes too much CO 2 from the atmosphere, the atmosphere will cool. • If the carbon cycle generates too much CO 2 the atmosphere will get warmer. • Even slight changes in the cycle can affect climate and help determine the types of life that can exist on various parts of the Earth.

The Carbon Cycle: How it Works • Terrestrial producers remove CO 2 from the atmosphere. • Aquatic producers remove CO 2 from the water. • All producers use photosynthesis to convert CO 2 into complex carbohydrates (like glucose) • The cells in consumers carry out aerobic respiration. They break down glucose and convert the glucose back to CO 2 for reuse by consumers. • The link between photosynthesis and aerobic respiration circulates carbon in the biosphere.

The Carbon Cycle: How • it. Some Works carbon atoms take a long time to recycle. • Over millions of years, buried deposits of dead plant matter and bacteria are compressed between layers of sediment, where they form carbon-containing fossil fuels. • This carbon is not released to the atmosphere as CO 2 for recycling until these fuels are extracted and burned. • In the past 50 years, we have extracted and burned fossil fuels that took millions of years to form.

The Carbon Cycle: The Role of Oceans • Some of the atmosphere’s carbon dioxide dissolves in ocean water and the ocean’s photosynthesizing producers remove some. • As the ocean water warms, some of the dissolved CO 2 returns to the atmosphere • Some ocean organisms build their shells and skeletons by using dissolved CO 2 molecules.

Effects of Human Activities on the Carbon Cycle • We alter the carbon cycle by… • Clear trees and plants that absorb CO 2 through photosynthesis faster than they can grow back • Add large amounts of CO 2 by burning fossil fuels and wood. • Increased concentrations of can enhance the planet’s natural greenhouse effect. • Global warming disrupts global food production and wildlife habitats, alter temperature and precipitation patterns, and raise the average sea level in various parts of the world.

Carbon may return to the air or water in 3 ways: Respiration- all living organisms undergo cellular respiration. They use oxygen to break down food; CO 2 is a byproduct of the reaction (exhaled). . Erosion- marine organisms use carbon to make shells, (calcium carbonate), when they die the calcium carbonate is broken down, CO 2 forms and is returned to the atmosphere. . Combustion- when carbon returns to the atmosphere through combustion or burning of fossil fuels. (Carbon is locked beneath the earth, dead organisms in sediment may gradually transform by heat and pressure into fossil fuels). Combustion of fossil fuels releases CO 2, which is a greenhouse gas.
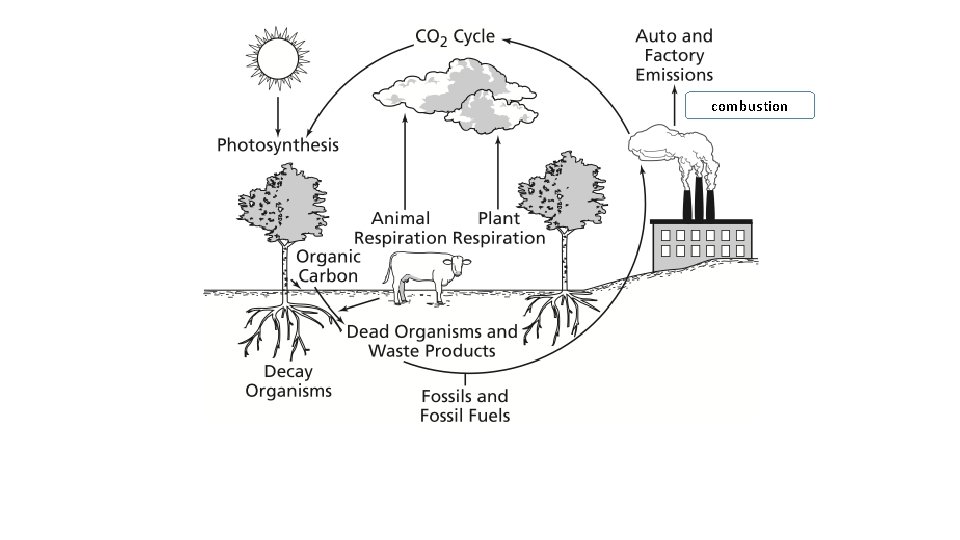
combustion
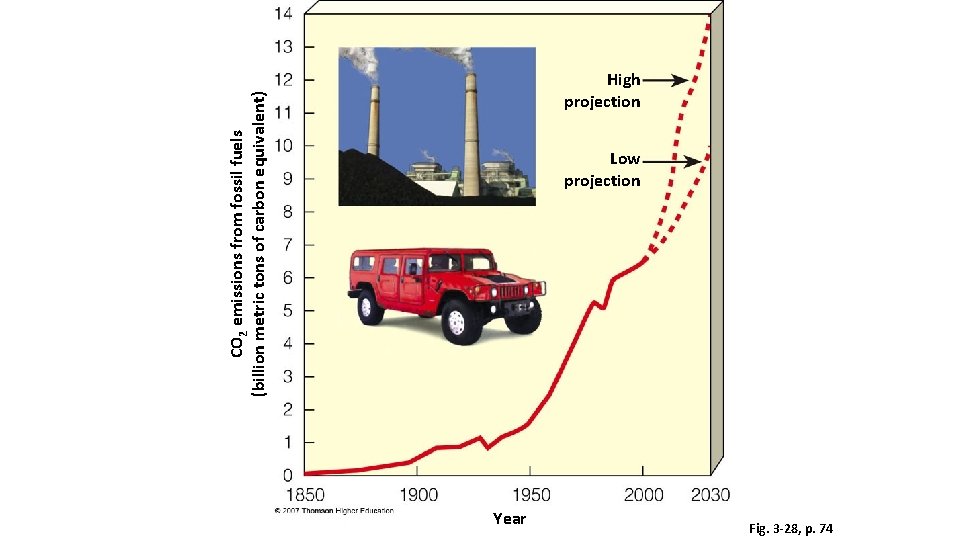
CO 2 emissions from fossil fuels (billion metric tons of carbon equivalent) High projection Low projection Year Fig. 3 -28, p. 74
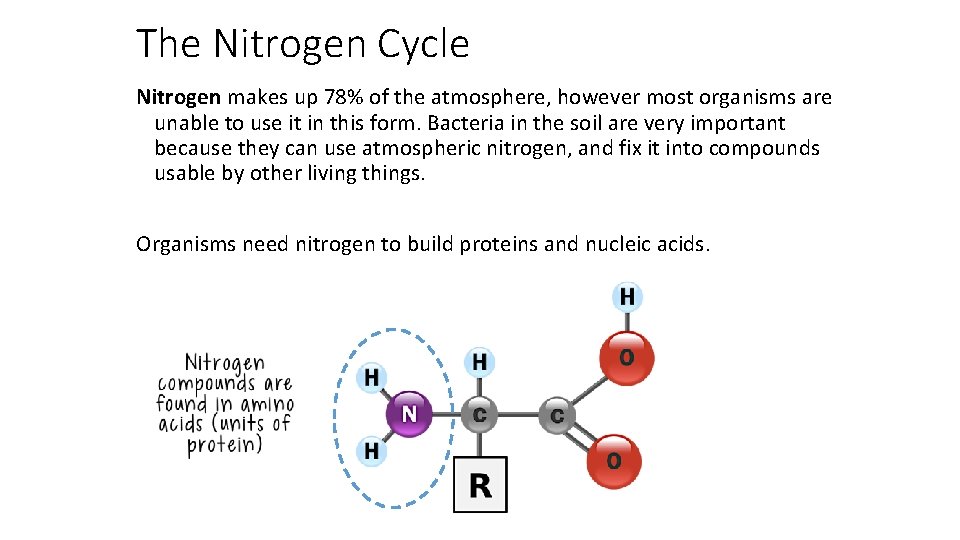
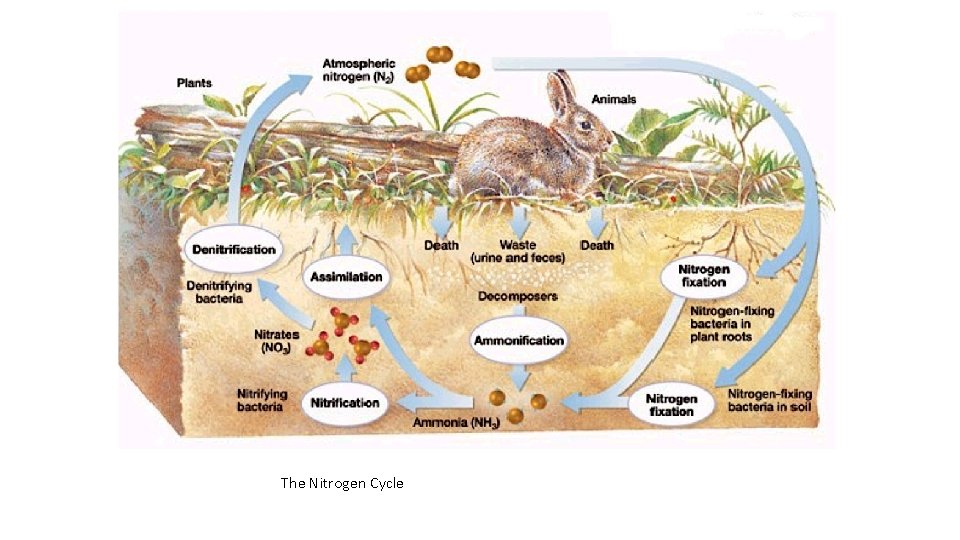
The Nitrogen Cycle Nitrogen makes up 78% of the atmosphere, however most organisms are unable to use it in this form. Bacteria in the soil are very important because they can use atmospheric nitrogen, and fix it into compounds usable by other living things. Organisms need nitrogen to build proteins and nucleic acids.
The Nitrogen Cycle • Two natural processes fix N 2 into useful compounds • Lightning • Nitrogen Cycle • Nitrogen-fixing bacteria in soil and aquatic environments convert (fix) gaseous nitrogen (N 2 ) into ammonia (NH 3) which is later converted into ammonium ions (NH 4+) that can be used by plants. • Ammonia not taken up by plants undergoes nitrification. Specialized soil bacteria convert the NH 3 and NH 4+ into nitrite ions (NO 2 -) which are toxic to plants, and then to nitrate (NO 3 -) ions which are taken up by the roots of plants. • Animals get their nitrogen by eating plants or planteating animals.
The Nitrogen Cycle • Plants and animals return nitrogen-rich organic compounds to the environment as wastes, cast-off particles, and through their bodies when they die. • In ammonificiation, large numbers of specialized decomposer bacteria convert organic material into simple nitrogen-containing inorganic compounds such as ammonia (NH 3) and water-soluble salts containing ammonium ions (NH 4+). • In denitrification, nitrogen leaves the soil as specialized bacteria in waterlogged soil and in the bottom sediments of lakes, oceans, swamps, and bogs to convert NH 3 and NH 4+ back into nitrite and nitrate ions, then into nitrogen gas (N 2) and nitrous oxide gas (N 2 O). These gases are released to the atmosphere to begin the nitrogen cycle again.
The Nitrogen Cycle
- Slides: 22