BIO 307 Bioengineering principles SPRING 2019 Lecture 10
BIO 307 - Bioengineering principles SPRING 2019 Lecture 10 Bio-molecular Engineering I: Biotechnology Lecturer: Jasmin Sutkovic 8. 5. 2019

Content Chapter 13: • • • Introduction Drug Delivery Tissue engineering Nano bio-technology More bio-molecular engineering Book chapter 13 (page 512 -547)
Introduction • Biomolecular engineering or Biotechnology • Study of the changes of chemical components in the biological system and developing methods to asses and modify these changes and interactions between molecules • Example: we take purified chemicals to treat pain such as aspirin and ibuprofen • Currently we move on to gene therapy – introducing new genes or editing the genome to achieve specific goal.
Introduction cont. . • In fact all chemicals in our body are somehow worth and their manipulation may result in great achievements. • Therefore, the understanding of their roles and interaction among each other is crucial for their successful manipulation • Humans often try to heal or treat diseases with chemicals (artificially produced molecules) and the Drug delivery process plays an important role. • Chemical engineering involves mathematical analysis of chemical reactions and transport phenomena; chemical engineering analysis is particularly well suited to problems involving multiple chemical species participating in multiple reactions.


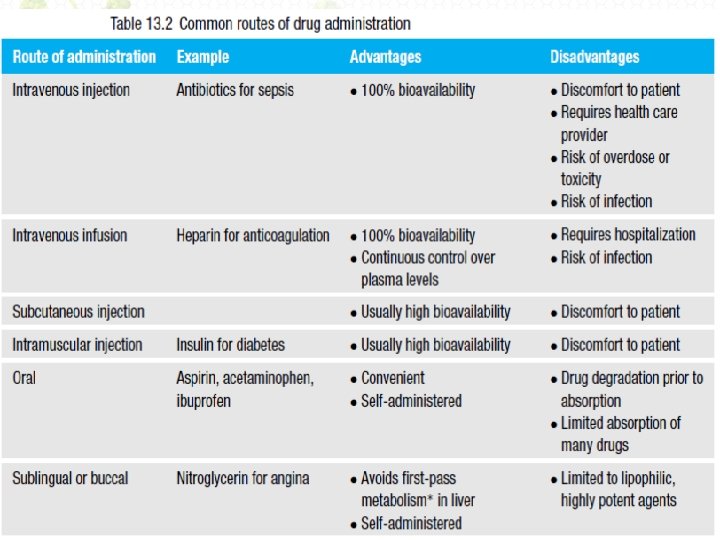
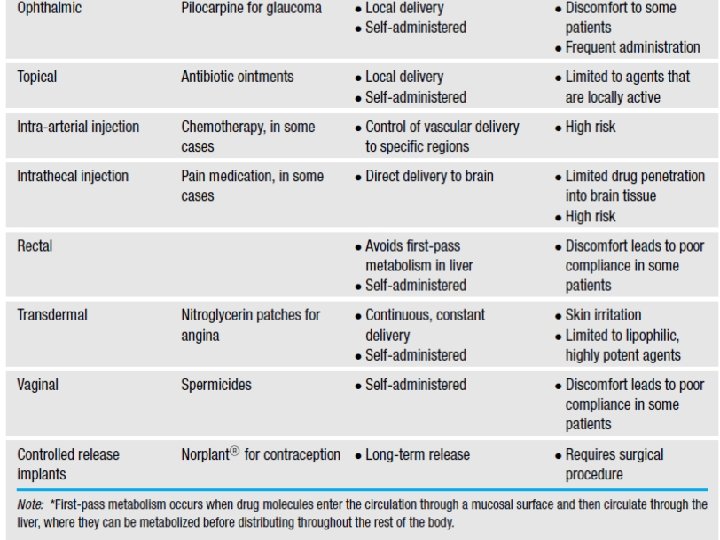
Drug delivery • Multiple forms of drug delivery exist – Pills, injections, lotions and suppositories • Oral intake is usually preferable • A variety of other modes of administration, less common than oral or injection, have evolved because of specific advantages for particular agents or certain diseases (see Table 13. 2).

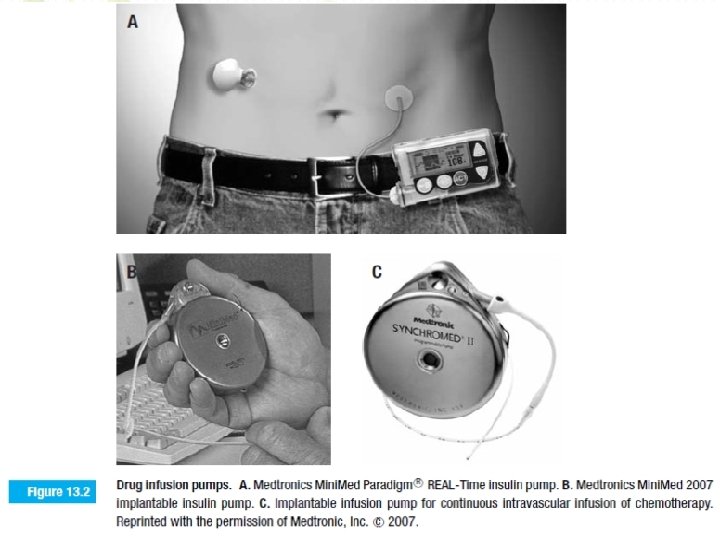
Are there better ways to deliver drugs? How can better methods be created? • Controlled drug-delivery systems can take a variety of forms like miniature mechanical pumps, polymer matrices microparticulates, externally applied transdermal patches, and transplanted, genetically engineered cells. • The most obvious approach for controlled drug delivery involves miniaturization of the familiar infusion system. • Mechanical pumps, either totally implantable or requiring catheters, have been used to deliver insulin, anticoagulants, analgesics, and cancer chemotherapy (Figure 13. 2).

Nondegradable polymeric reservoirs and matrices • The first controlled release polymer systems were based on silicone elastomers. • In 1964, researchers recognized that certain dye molecules could diffuse through the walls of silicone tubing. This observation led to the development of reservoir drug-delivery systems: hollow silicone tubes filled with a liquid suspension of the drug of interest.
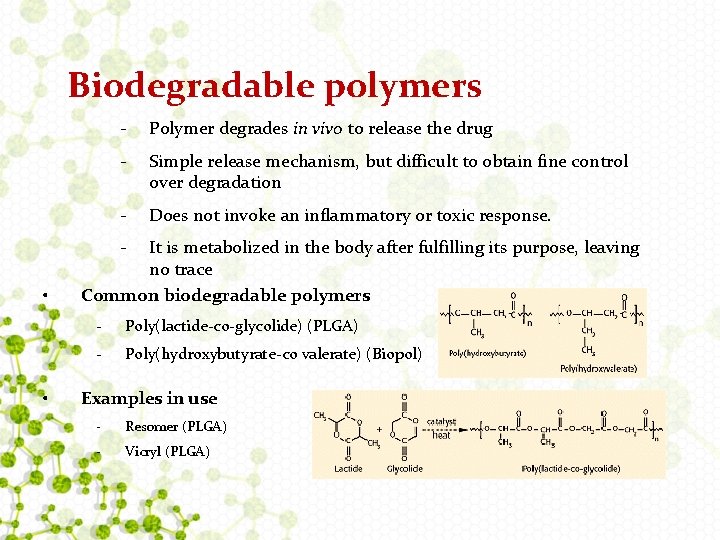
Biodegradable polymers - Polymer degrades in vivo to release the drug - Simple release mechanism, but difficult to obtain fine control over degradation - Does not invoke an inflammatory or toxic response. - • • It is metabolized in the body after fulfilling its purpose, leaving no trace Common biodegradable polymers - Poly(lactide-co-glycolide) (PLGA) - Poly(hydroxybutyrate-co valerate) (Biopol) Examples in use - Resomer (PLGA) - Vicryl (PLGA)
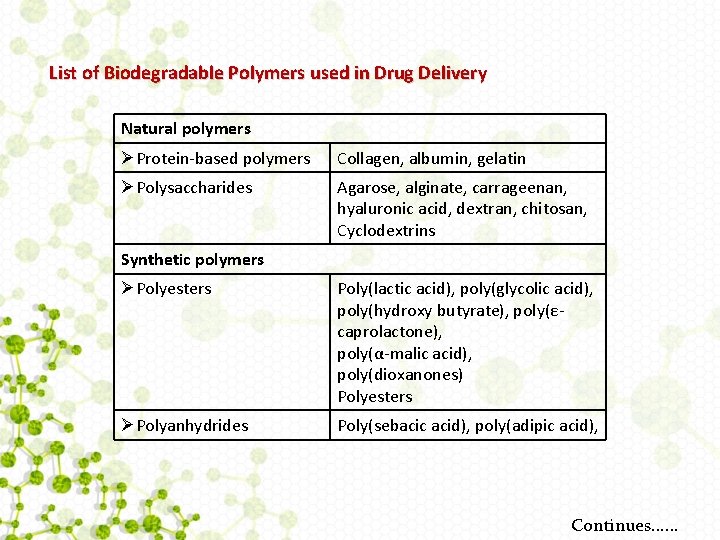
List of Biodegradable Polymers used in Drug Delivery Natural polymers ØProtein-based polymers Collagen, albumin, gelatin ØPolysaccharides Agarose, alginate, carrageenan, hyaluronic acid, dextran, chitosan, Cyclodextrins Synthetic polymers ØPolyesters Poly(lactic acid), poly(glycolic acid), poly(hydroxy butyrate), poly(εcaprolactone), poly(α-malic acid), poly(dioxanones) Polyesters ØPolyanhydrides Poly(sebacic acid), poly(adipic acid), Continues. . .
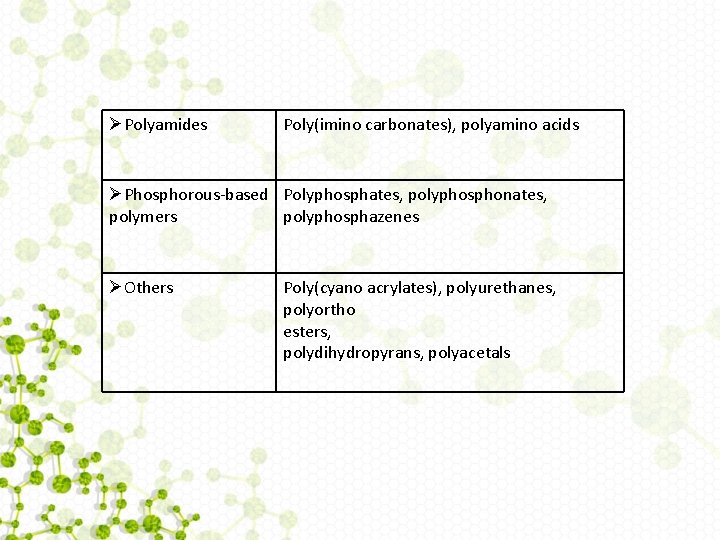
ØPolyamides Poly(imino carbonates), polyamino acids ØPhosphorous-based Polyphosphates, polyphosphonates, polymers polyphosphazenes ØOthers Poly(cyano acrylates), polyurethanes, polyortho esters, polydihydropyrans, polyacetals
Polymeric drug delivery system devices �Particulate systems �Nanoparticles � Nanocapsules � Nanospheres �Microparticles � Microspheres � Microcapsules
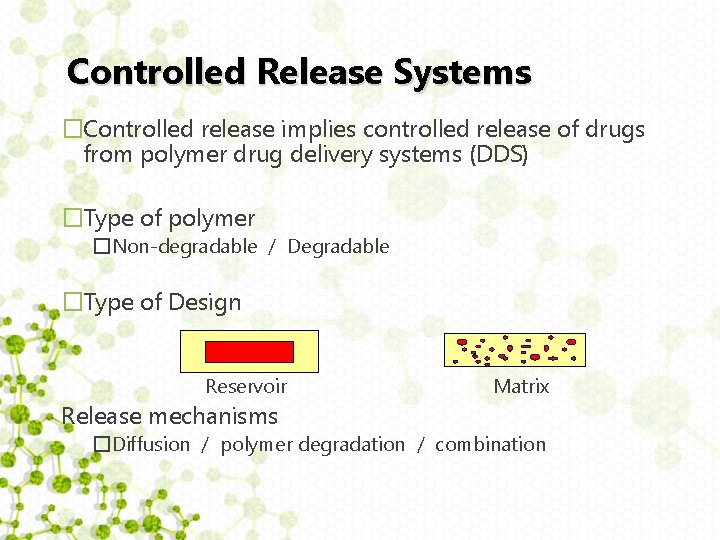
Controlled Release Systems �Controlled release implies controlled release of drugs from polymer drug delivery systems (DDS) �Type of polymer �Non-degradable / Degradable �Type of Design Reservoir Release mechanisms Matrix �Diffusion / polymer degradation / combination
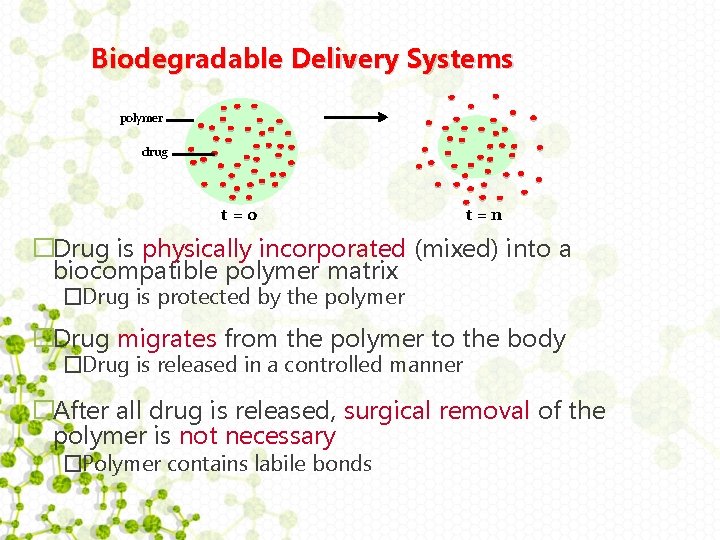
Biodegradable Delivery Systems polymer drug t=0 t=n �Drug is physically incorporated (mixed) into a biocompatible polymer matrix �Drug is protected by the polymer �Drug migrates from the polymer to the body �Drug is released in a controlled manner �After all drug is released, surgical removal of the polymer is not necessary �Polymer contains labile bonds
Controlled delivery of proteins and other macromolecules • Recombinant proteins and polypeptides are now produced in large quantities by the biotechnology industry. • Many of these novel molecules—like recombinant tissue plasminogen activator, human insulin, and human growth hormone already have important applications in human health.

Protein drugs? • Protein drugs are difficult to use in humans because they are either : 1) eliminated very quickly when introduced into the body or 2) toxic when delivered systemically at the doses required to achieve a local effect. • Controlled release polymers may overcome these problems by – 1) slowly releasing the protein into the blood over a long period or – 2) releasing protein into a local tissue site, thus sparing systemic exposure.
Genetically engineered cells for controlled drug delivery • Future drug-delivery systems will probably use sophisticated biological components that allow selfregulation of drug release. • Therefore, advanced polymer systems that provide temporal control over protein release or selfregulation are being studied. • Many of these advanced systems use biological components, like enzymes or antibodies, as the signaling element for regulation of release
Examples • Cells harvested from a patient could be genetically modified to increase production of a protein of interest; genes enhancing the cell’s ability to control protein expression could also be added. • For example, hydrogel materials have been used to encapsulate engineered cells as a means of isolating them from direct contact with cells of the immune system (Figure 13. 6).

Tissue engineering • Tissue engineering combines knowledge from the biological sciences with materials science and engineering to accomplish several goals: – to quantify structure–function relationships in normal and pathological tissues – to develop new approaches – to repair tissues, and – to develop replacements for tissues.

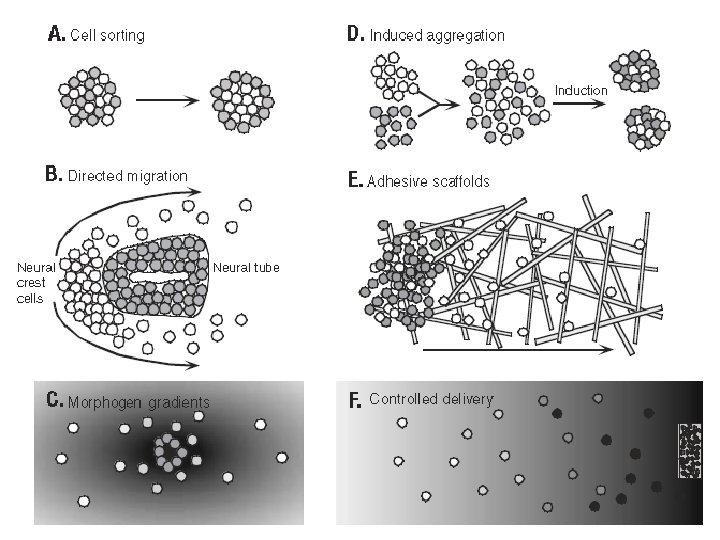
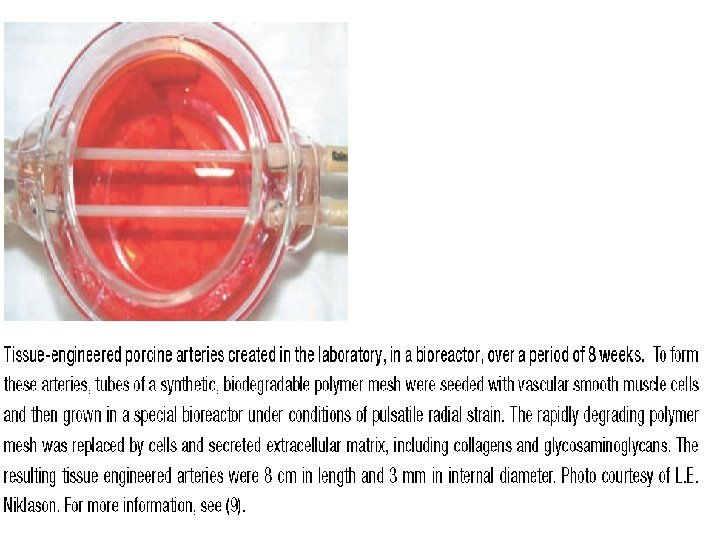
How can tissue development in culture be manipulated? • Several laboratory techniques • Central among these new strategies is the idea, first proposed less than 20 years ago, that synthetic materials can serve as degradable templates for tissue regeneration (Figure 13. 7 e).

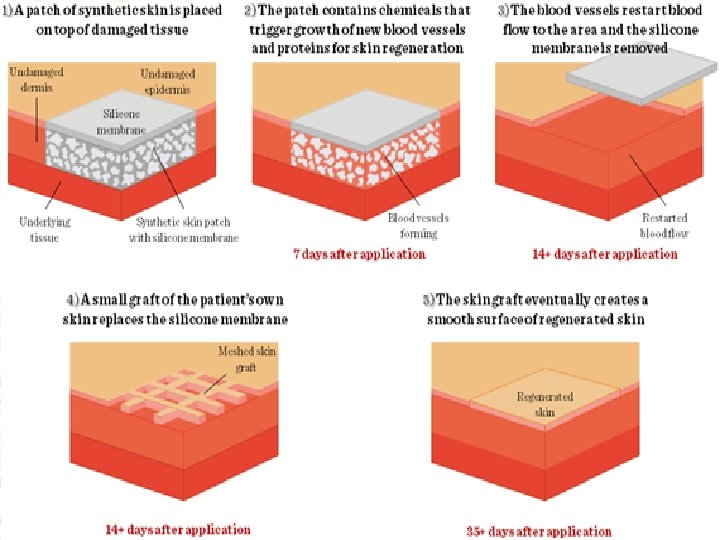
Artificial skin (Skin Graft) Newer approaches to artificial skin involve spider silk and spray-on skin
Nanobiotechnology • Materials of nanoscale size made it possible to produce nano devices and technology for various purposes • Biological function depends heavily on units that have nanoscale dimensions, such as viruses, ribosomes, molecular motors, and components of the extracellular matrix

Nanoscale delivery systems • Although there a variety of ways of achieving nanoscale delivery systems, including selfassembling systems based on liposomes or micelles, the most stable systems are miniaturized versions of the synthetic materials that have already been used in drug-delivery applications. • Construction of such systems is usually accomplished with degradable polymers such as PLGA.
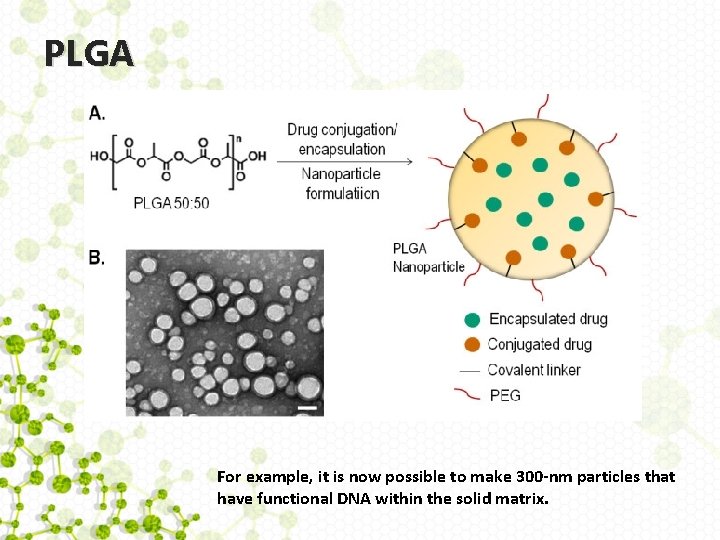
PLGA For example, it is now possible to make 300 -nm particles that have functional DNA within the solid matrix.

• See you next time…
- Slides: 32