BIO 101 Chapter 3 Organic Chemistry Structural formula
BIO 101 Chapter 3 Organic Chemistry
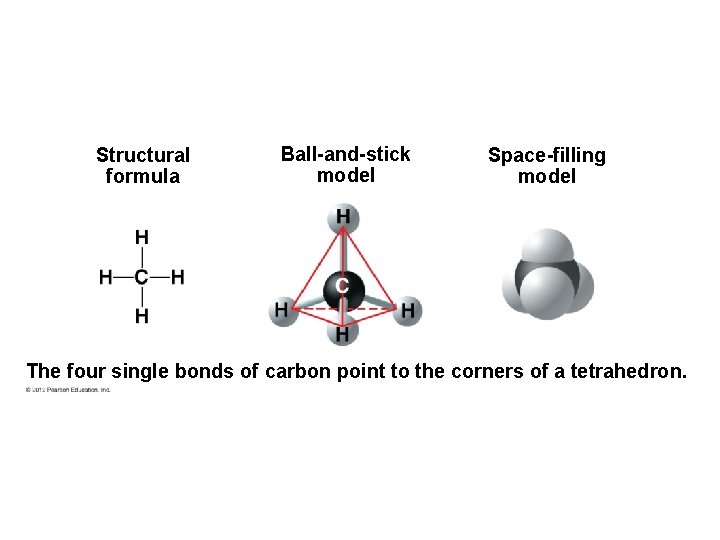
Structural formula Ball-and-stick model Space-filling model The four single bonds of carbon point to the corners of a tetrahedron.
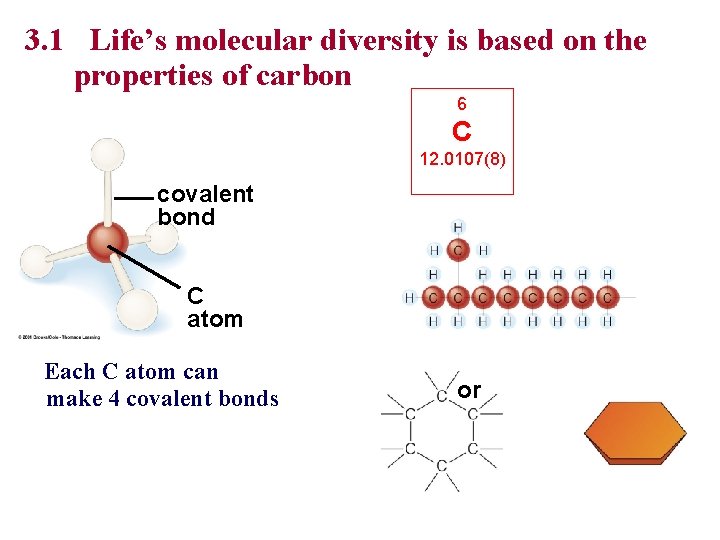
3. 1 Life’s molecular diversity is based on the properties of carbon 6 C 12. 0107(8) covalent bond C atom Each C atom can make 4 covalent bonds or
3. 2 A few chemical groups are key to the functioning of biological molecules § An organic compound has unique properties that depend upon the – size and shape of the molecule and – groups of atoms (functional groups) attached to it. § A functional group affects a biological molecule’s function in a characteristic way. © 2012 Pearson Education, Inc.
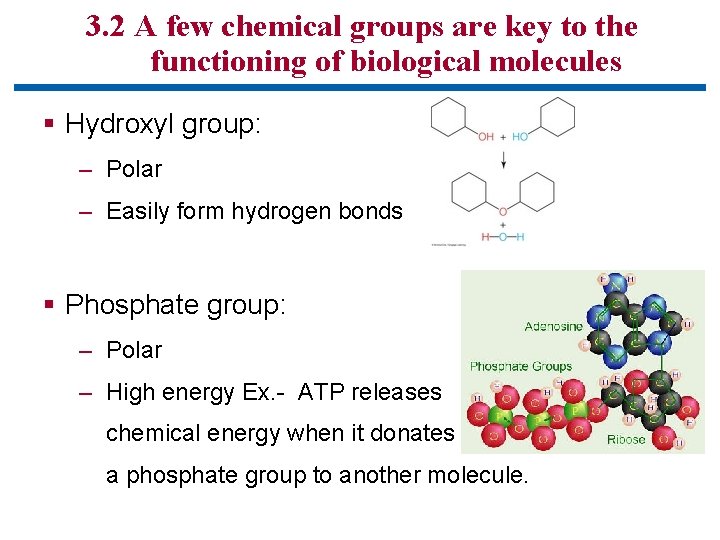
3. 2 A few chemical groups are key to the functioning of biological molecules § Hydroxyl group: – Polar – Easily form hydrogen bonds § Phosphate group: – Polar – High energy Ex. - ATP releases chemical energy when it donates a phosphate group to another molecule.
3. 3 Cells make a huge number of large molecules from a limited set of small molecules § There are 4 Classes of Molecules) important to organisms: (aka- 4 Major Groups of Organic Compounds) 1. 2. 3. 4. – They are often called ______ because of their large size. – They are also called _____ because they are made from identical building blocks strung together. – The building blocks of polymers are called ______. © 2012 Pearson Education, Inc.
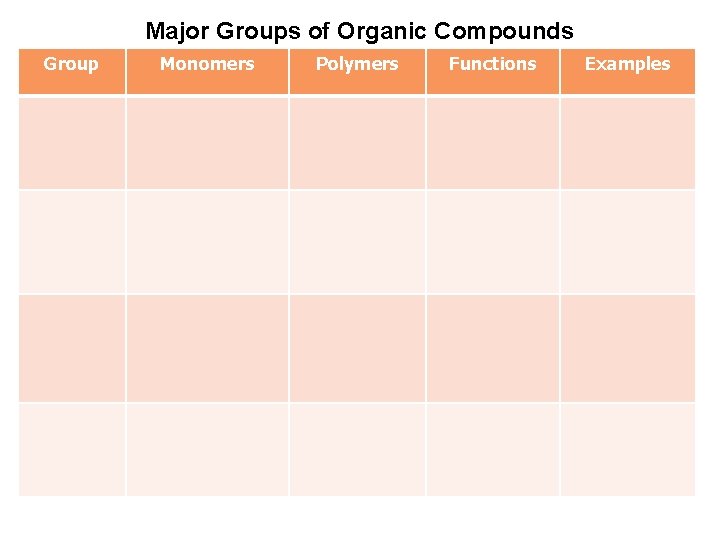
Major Groups of Organic Compounds Group Monomers Polymers Functions Examples

Carbohydrates for energy
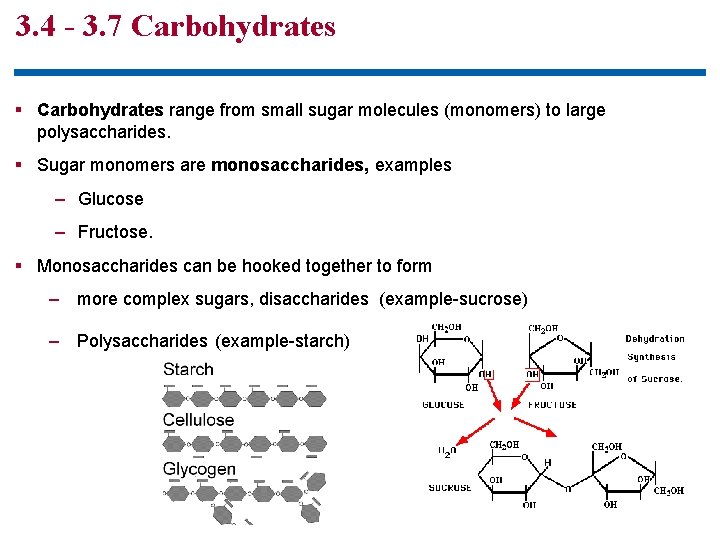
3. 4 - 3. 7 Carbohydrates § Carbohydrates range from small sugar molecules (monomers) to large polysaccharides. § Sugar monomers are monosaccharides, examples – Glucose – Fructose. § Monosaccharides can be hooked together to form – more complex sugars, disaccharides (example-sucrose) – Polysaccharides (example-starch)
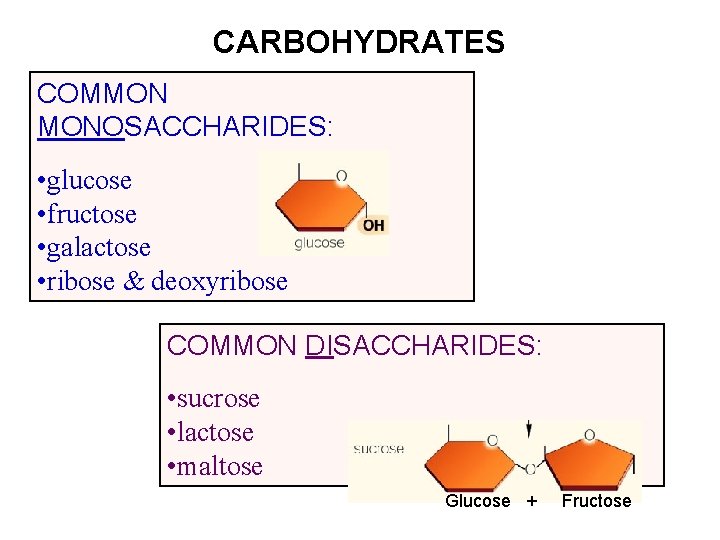
CARBOHYDRATES COMMON MONOSACCHARIDES: • glucose • fructose • galactose • ribose & deoxyribose COMMON DISACCHARIDES: • sucrose • lactose • maltose Glucose + Fructose
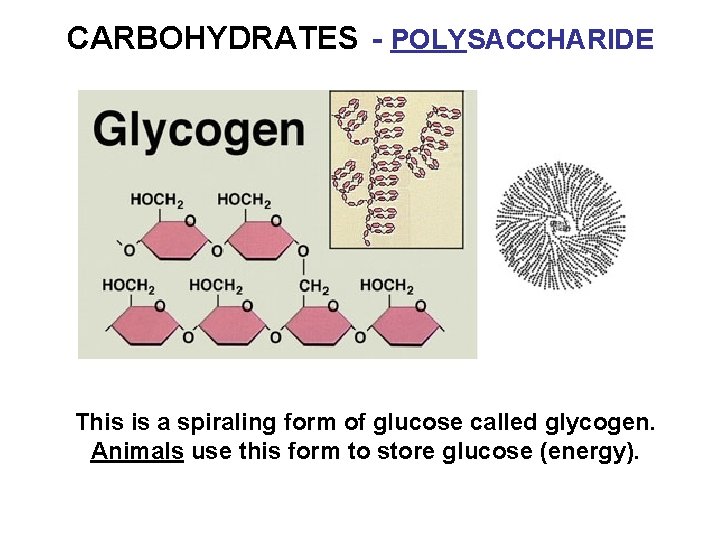
CARBOHYDRATES - POLYSACCHARIDE This is a spiraling form of glucose called glycogen. Animals use this form to store glucose (energy).
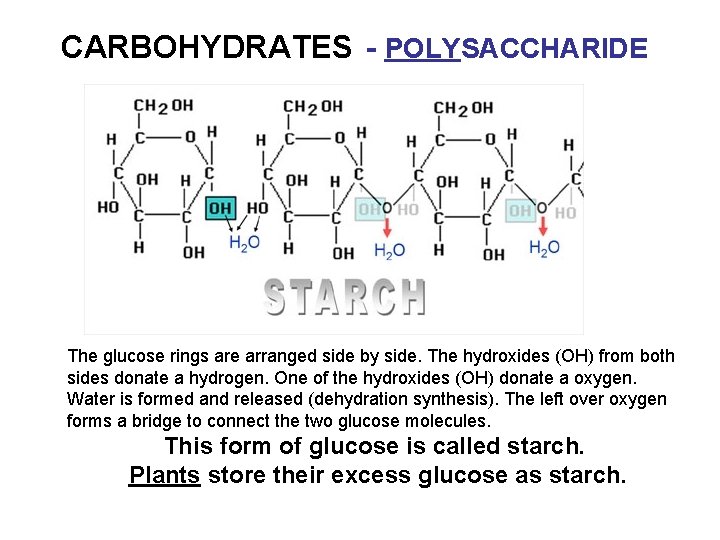
CARBOHYDRATES - POLYSACCHARIDE The glucose rings are arranged side by side. The hydroxides (OH) from both sides donate a hydrogen. One of the hydroxides (OH) donate a oxygen. Water is formed and released (dehydration synthesis). The left over oxygen forms a bridge to connect the two glucose molecules. This form of glucose is called starch. Plants store their excess glucose as starch.
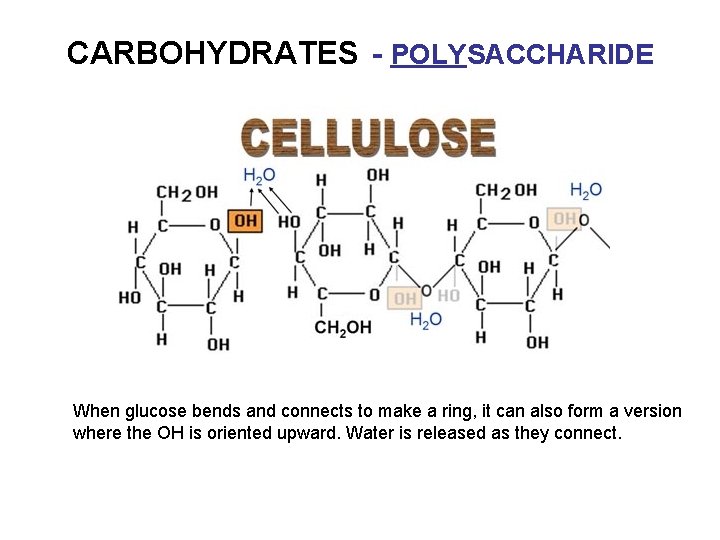
CARBOHYDRATES - POLYSACCHARIDE When glucose bends and connects to make a ring, it can also form a version where the OH is oriented upward. Water is released as they connect.
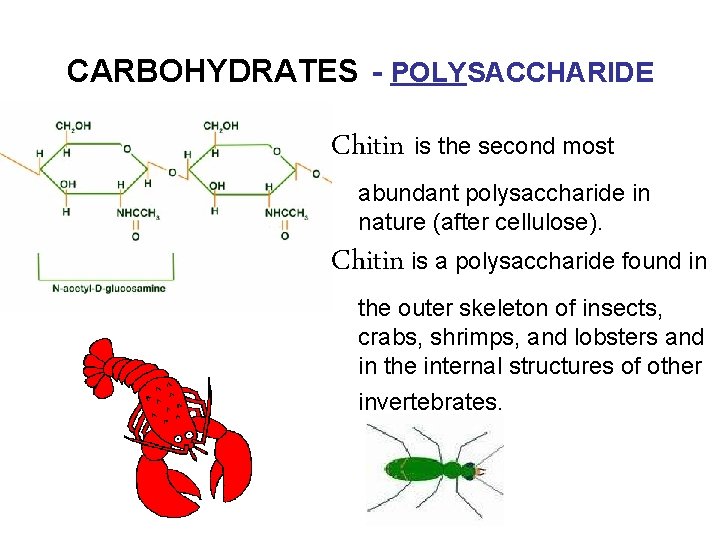
CARBOHYDRATES - POLYSACCHARIDE Chitin is the second most abundant polysaccharide in nature (after cellulose). Chitin is a polysaccharide found in the outer skeleton of insects, crabs, shrimps, and lobsters and in the internal structures of other invertebrates.
3. 7 Polysaccharides are long chains of sugar units § Polysaccharides (starch, cellulose, chitin, glycogen) are – macromolecules and – polymers composed of thousands of monosaccharides. § Polysaccharides may function as – storage molecules (starch, glycogen) – structural compounds (cellulose, chitin) © 2012 Pearson Education, Inc.
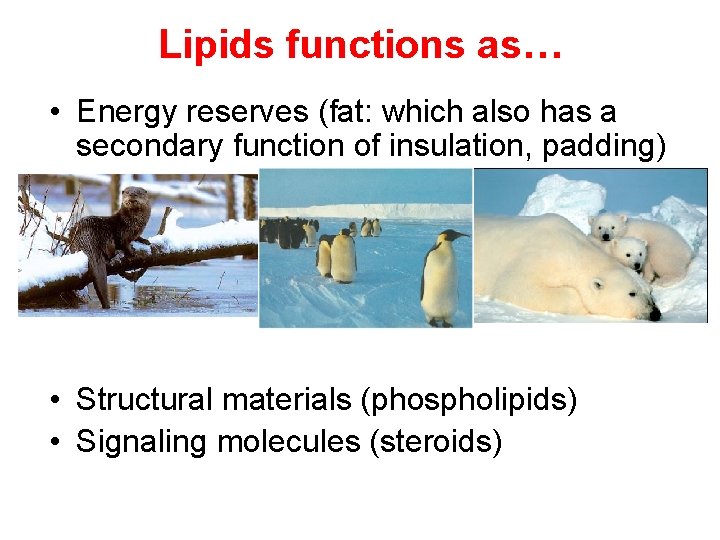
Lipids functions as… • Energy reserves (fat: which also has a secondary function of insulation, padding) • Structural materials (phospholipids) • Signaling molecules (steroids)
3. 8 Fats are lipids that are mostly energy-storage molecules § Lipids – are water insoluble (hydrophobic, or water-fearing) compounds, – are important in long-term energy storage, – contain twice as much energy as a polysaccharide, and – consist mainly of carbon and hydrogen atoms linked by nonpolar covalent bonds. – are not huge molecules – not built from monomers. © 2012 Pearson Education, Inc.
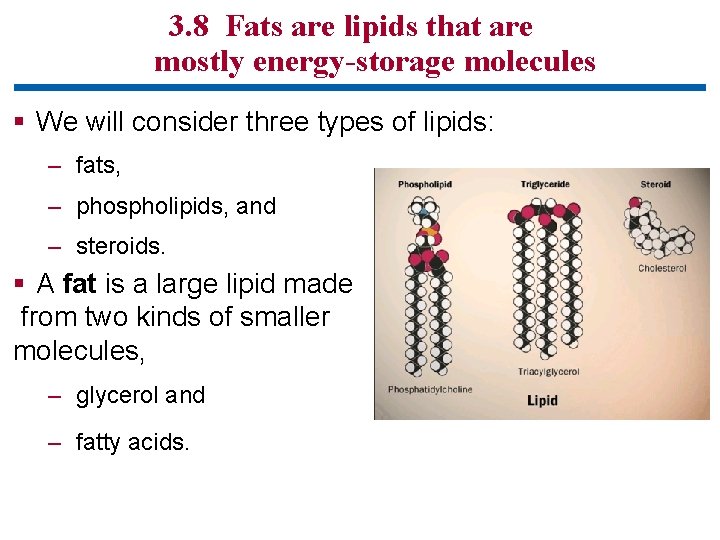
3. 8 Fats are lipids that are mostly energy-storage molecules § We will consider three types of lipids: – fats, – phospholipids, and – steroids. § A fat is a large lipid made from two kinds of smaller molecules, – glycerol and – fatty acids.
3. 9 Phospholipids and steroids are important lipids with a variety of functions § Phospholipids are – structurally similar to fats and – the major component of all cells. § Phospholipids are structurally similar to fats. – Fats contain three fatty acids attached to glycerol. – Phospholipids contain two fatty acids attached to glycerol. © 2012 Pearson Education, Inc.
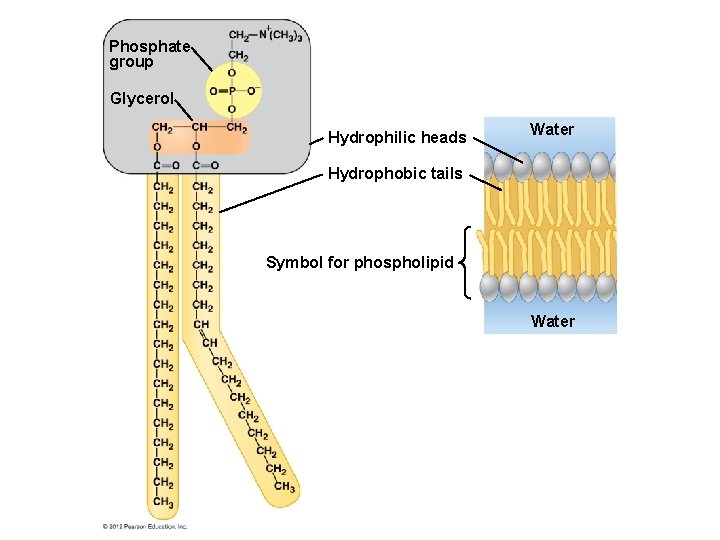
Phosphate group Glycerol Hydrophilic heads Water Hydrophobic tails Symbol for phospholipid Water
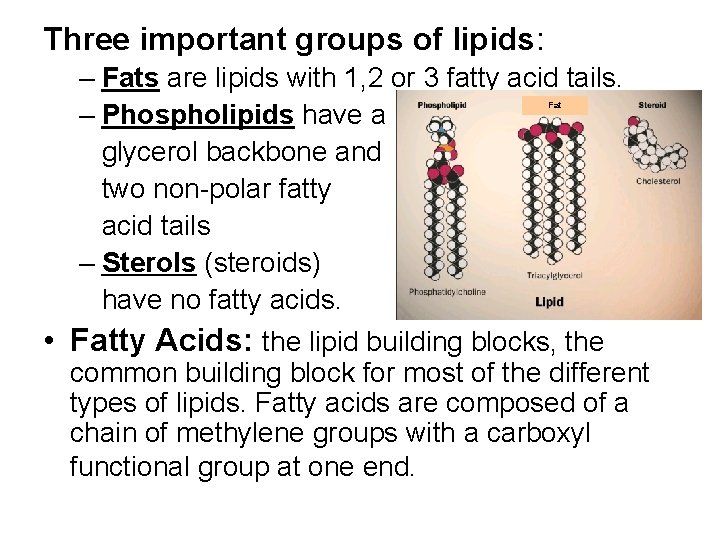
Three important groups of lipids: – Fats are lipids with 1, 2 or 3 fatty acid tails. – Phospholipids have a glycerol backbone and two non-polar fatty acid tails – Sterols (steroids) have no fatty acids. • Fatty Acids: the lipid building blocks, the common building block for most of the different types of lipids. Fatty acids are composed of a chain of methylene groups with a carboxyl functional group at one end. Fat
Protein Diversity Amino acids build proteins
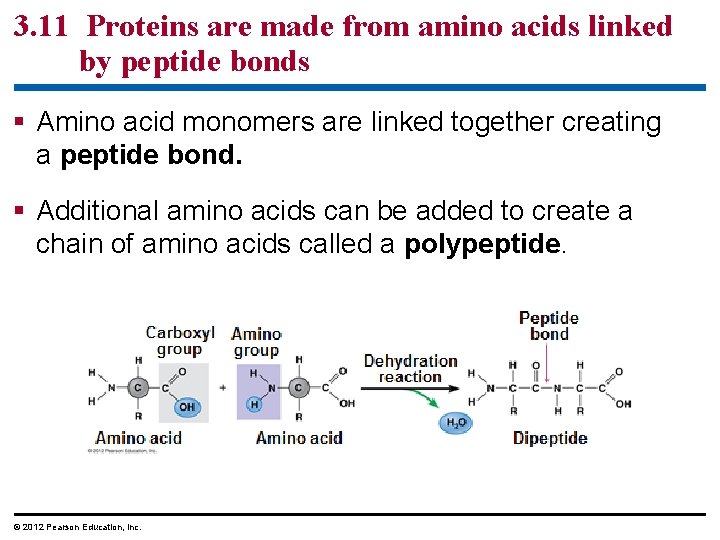
3. 11 Proteins are made from amino acids linked by peptide bonds § Amino acid monomers are linked together creating a peptide bond. § Additional amino acids can be added to create a chain of amino acids called a polypeptide. © 2012 Pearson Education, Inc.
3. 12 A protein’s specific shape determines its function § Probably the most important role for proteins is as an enzyme. Enzymes are proteins that – serve as metabolic catalysts and – regulate the chemical reactions within cells. © 2012 Pearson Education, Inc.
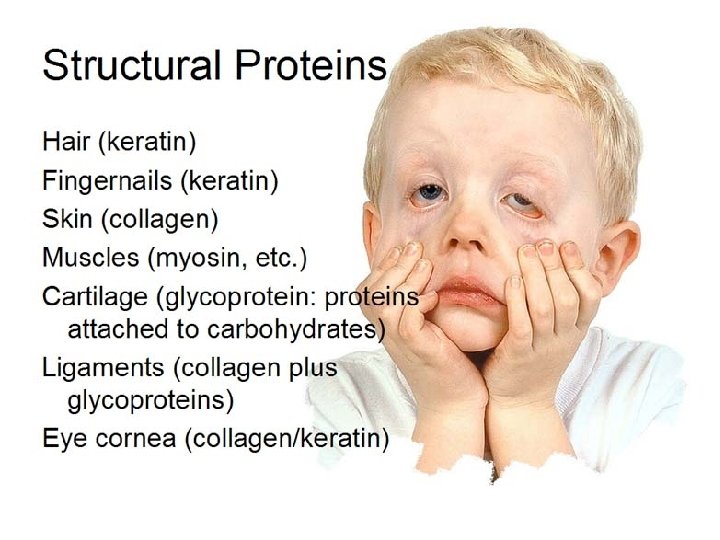
3. 12 A protein’s specific SHAPE determines its function § Other proteins functions: – Structural proteins provide associations between body parts. – Contractile proteins are found within muscle. – Defensive proteins include antibodies of the immune system. – Signal proteins are best exemplified by hormones and other chemical messengers. – Receptor proteins transmit signals into cells. – Transport proteins carry oxygen. – Storage proteins serve as a source of amino acids for developing embryos. © 2012 Pearson Education, Inc.
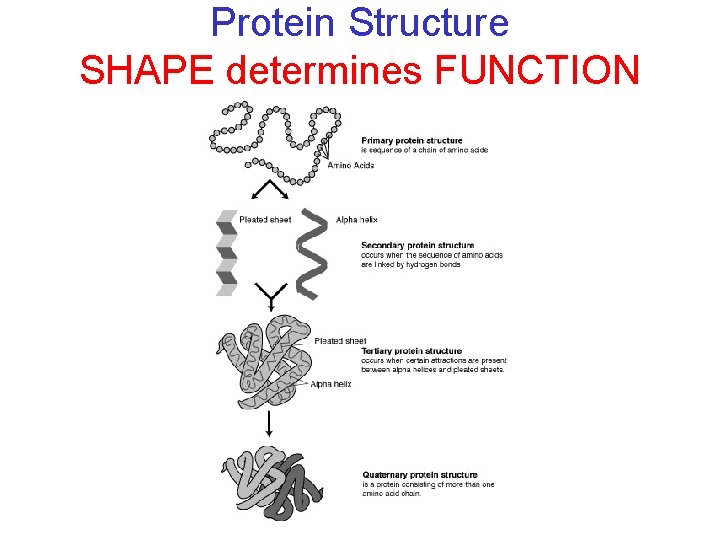
Protein Structure SHAPE determines FUNCTION
3. 12 A protein’s specific shape determines its function § If a protein’s shape is altered, it can no longer function. § In the process of denaturation, a polypeptide chain – unravels, – Protein loses its ______, and – Therefore loses its ________. § Proteins can be denatured by changes in salt concentration, p. H, or by high heat. © 2012 Pearson Education, Inc.
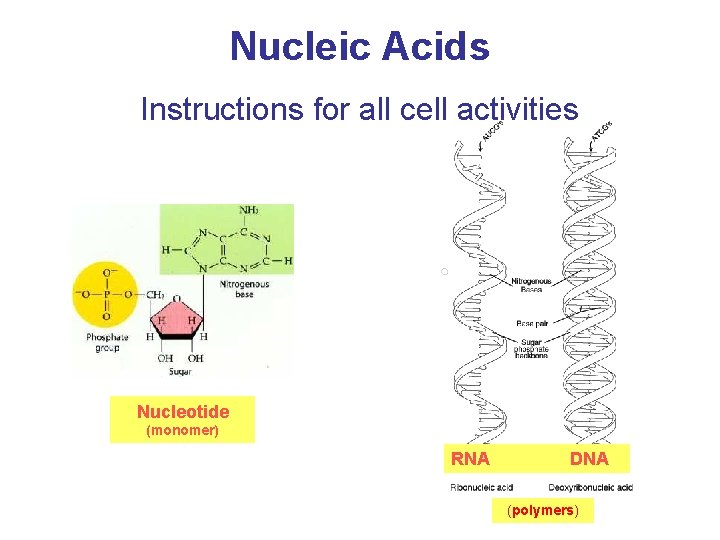
Nucleic Acids Instructions for all cell activities Nucleotide (monomer) RNA DNA (polymers)
3. 15 Nucleic acids are polymers of nucleotides § Genes consist of DNA (deoxyribonucleic acid) § RNA (ribonucleic acid) directs the synthesis of proteins from instructions in the DNA. § Both DNA and RNA are made up of nucleotides which have three parts: – a five-carbon sugar called ____ in RNA and _________ in DNA, – a phosphate group, and – a nitrogenous base. © 2012 Pearson Education, Inc.
You should now be able to 1. Describe the properties of carbon that allow for diverse molecules. 2. Name the 4 classes or groups of molecules that are important to life. 3. Explain how a cell can make a variety of large molecules from a small set of molecules. 4. Define monosaccharides, disaccharides, and polysaccharides. Explain the functions of carbohydrates. © 2012 Pearson Education, Inc.
You should now be able to 5. Define lipids, phospholipids, and steroids and explain their functions. 6. Describe the chemical structure of proteins and their diverse functions. 7. Describe the chemical structure of nucleic acids and how they relate to inheritance. © 2012 Pearson Education, Inc.
- Slides: 32