BinaryTernary Acid Nomenclature Binary Acids are acids made
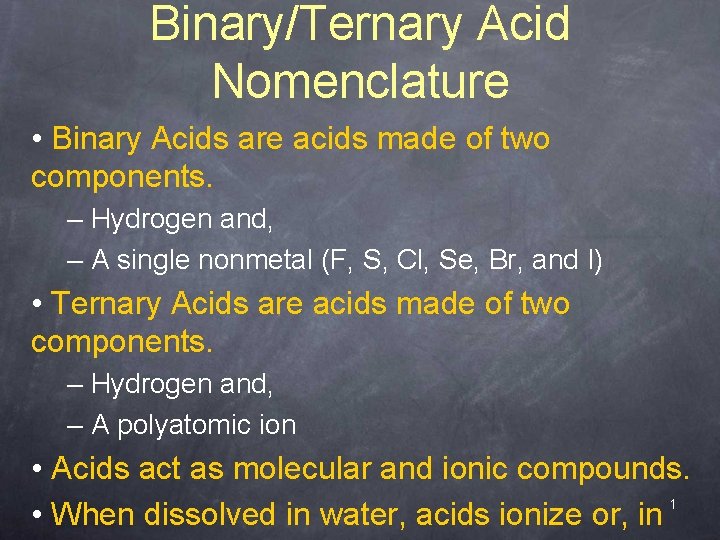
Binary/Ternary Acid Nomenclature • Binary Acids are acids made of two components. – Hydrogen and, – A single nonmetal (F, S, Cl, Se, Br, and I) • Ternary Acids are acids made of two components. – Hydrogen and, – A polyatomic ion • Acids act as molecular and ionic compounds. 1 • When dissolved in water, acids ionize or, in
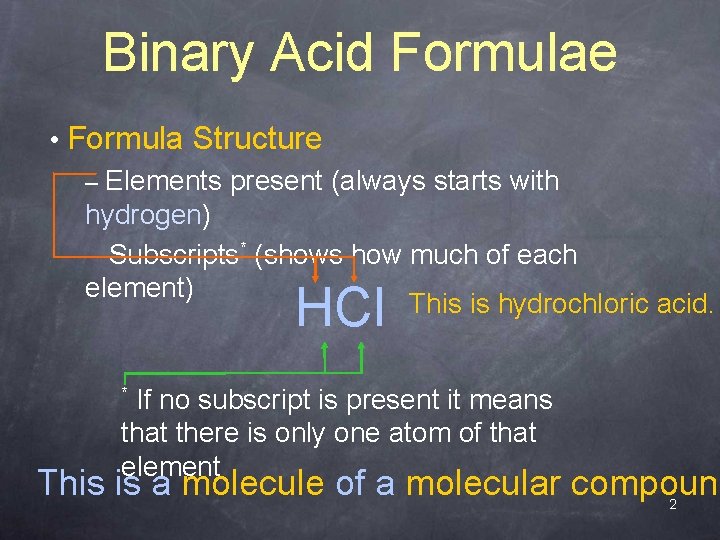
Binary Acid Formulae • Formula Structure – Elements present (always starts with hydrogen) – Subscripts* (shows how much of each element) This is hydrochloric acid. HCl If no subscript is present it means that there is only one atom of that element * This is a molecule of a molecular compound 2
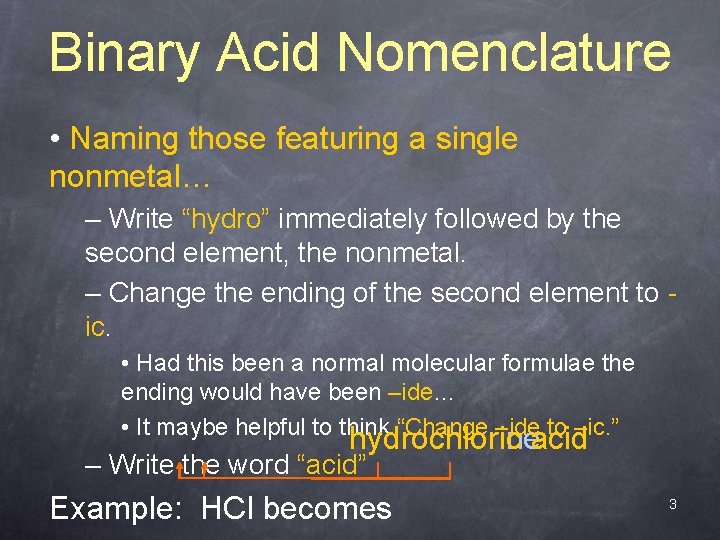
Binary Acid Nomenclature • Naming those featuring a single nonmetal… – Write “hydro” immediately followed by the second element, the nonmetal. – Change the ending of the second element to ic. • Had this been a normal molecular formulae the ending would have been –ide… • It maybe helpful to think “Change –ide to –ic. ” hydrochlorineacid hydrochloric – Write the word “acid” Example: HCl becomes 3
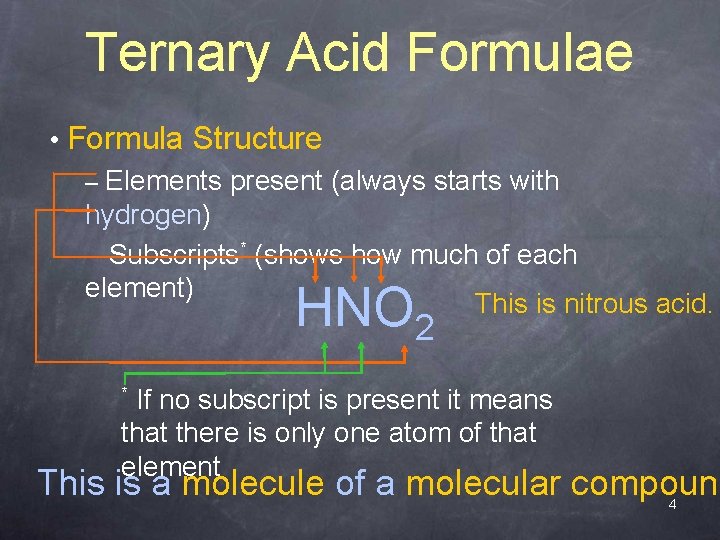
Ternary Acid Formulae • Formula Structure – Elements present (always starts with hydrogen) – Subscripts* (shows how much of each element) This is nitrous acid. HNO 2 If no subscript is present it means that there is only one atom of that element * This is a molecule of a molecular compound 4
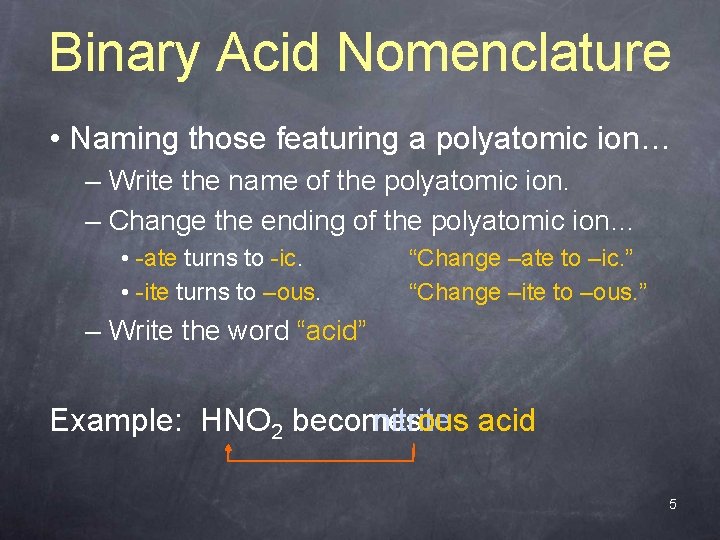
Binary Acid Nomenclature • Naming those featuring a polyatomic ion… – Write the name of the polyatomic ion. – Change the ending of the polyatomic ion… • -ate turns to -ic. • -ite turns to –ous. “Change –ate to –ic. ” “Change –ite to –ous. ” – Write the word “acid” nitrous acid nitrite Example: HNO 2 becomes 5
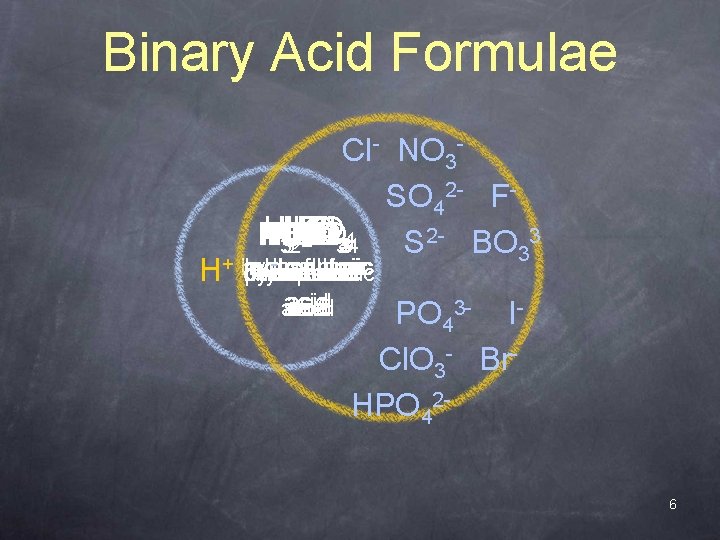
Binary Acid Formulae Cl- NO 3 SO 42 - FH H HCl HF PO S H HCl. O HNO HBr HI PO BO SO 3 2 332 34 344 S 2 - BO 33 -sulfuric hydrofluoric hydrochloric phosphoric H+ hydrosulfuric hydrobromic chloric phosphoric hydroiodic boric nitric acid acid PO 43 - ICl. O 3 - Br. HPO 426
- Slides: 6