Binary Phase Diagrams GLY 4200 Fall 2012 1

Binary Phase Diagrams GLY 4200 Fall, 2012 1
Binary Diagrams • Binary diagrams have two components • We therefore usually choose to plot both T (temperature) and X (composition) with pressure held constant • P-X (T fixed) or P-T (X fixed) are also possible 2
Binary System Examples • Binary solid solution - olivine, plagioclase feldspar • Binary eutectic with congruent melting potassium feldspar - silica • Binary peritectic and eutectic with solid to solid conversion - leucite - Potassium feldspar - silica • Binary minimum melting point - potassium feldspar - albite • Binary minimum melting point with solvus potassium feldspar - albite 3
Phase Rule for Binary Systems § f=c-p+2=2 -p+2=4 -p • If two phases are present, there are two degrees of freedom (both T and X) • If three phases are present, there is one degree of freedom (either T or X) 4
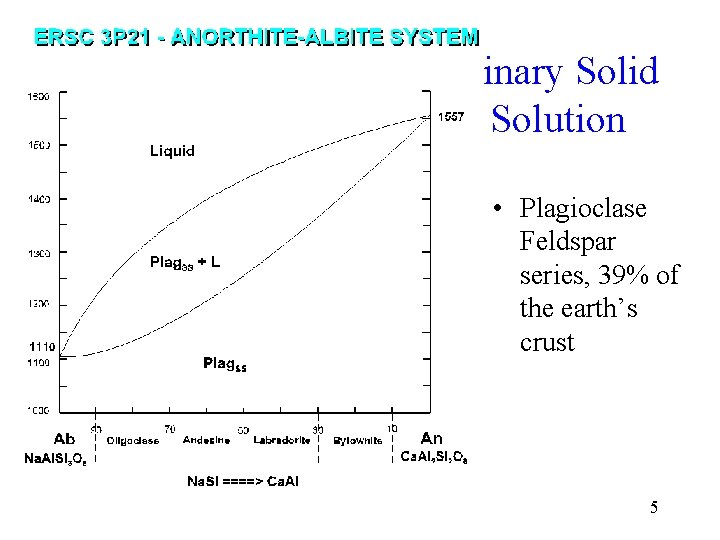
Binary Solid Solution • Plagioclase Feldspar series, 39% of the earth’s crust 5
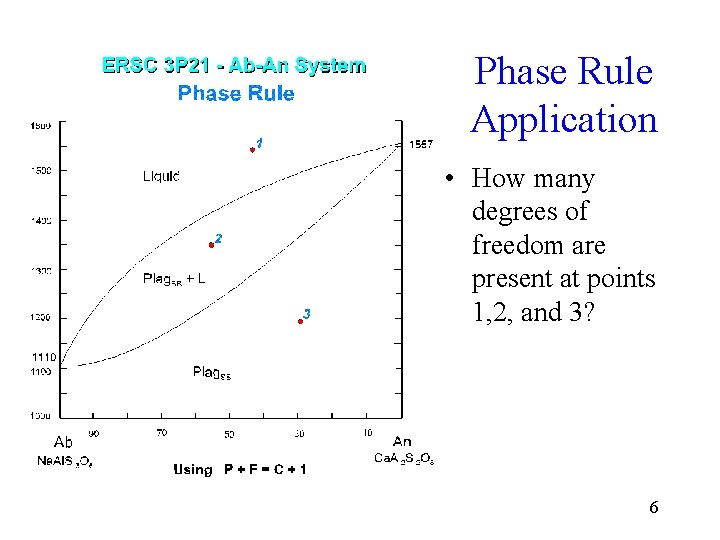
Phase Rule Application • How many degrees of freedom are present at points 1, 2, and 3? 6
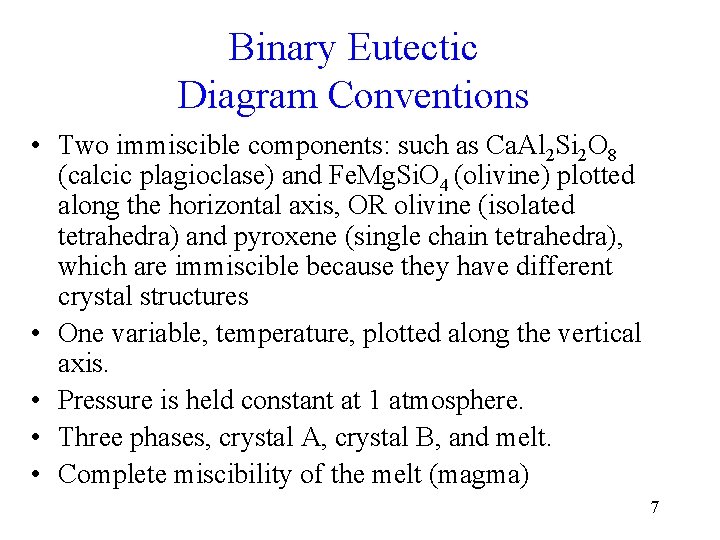
Binary Eutectic Diagram Conventions • Two immiscible components: such as Ca. Al 2 Si 2 O 8 (calcic plagioclase) and Fe. Mg. Si. O 4 (olivine) plotted along the horizontal axis, OR olivine (isolated tetrahedra) and pyroxene (single chain tetrahedra), which are immiscible because they have different crystal structures • One variable, temperature, plotted along the vertical axis. • Pressure is held constant at 1 atmosphere. • Three phases, crystal A, crystal B, and melt. • Complete miscibility of the melt (magma) 7

Binary Eutectic Diagram Assumptions • The system remains in equilibrium throughout its history, so that all reactions can take place and everything can come to stability • Everything in the original melt remains in communication throughout the crystallization process 8
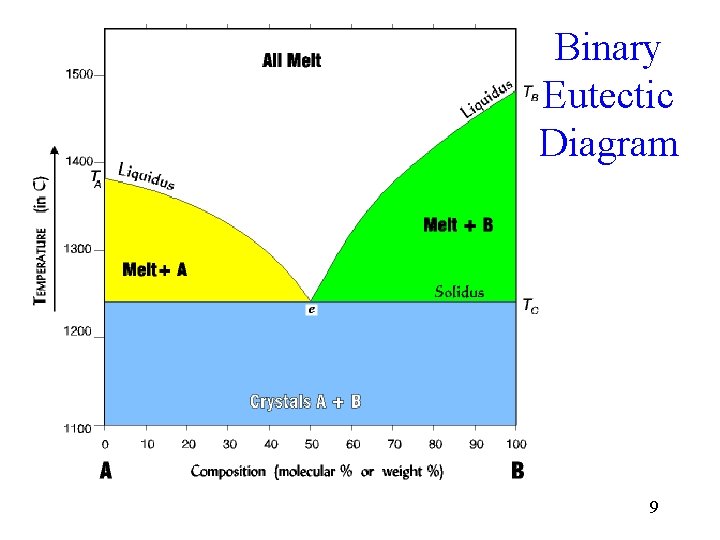
Binary Eutectic Diagram 9
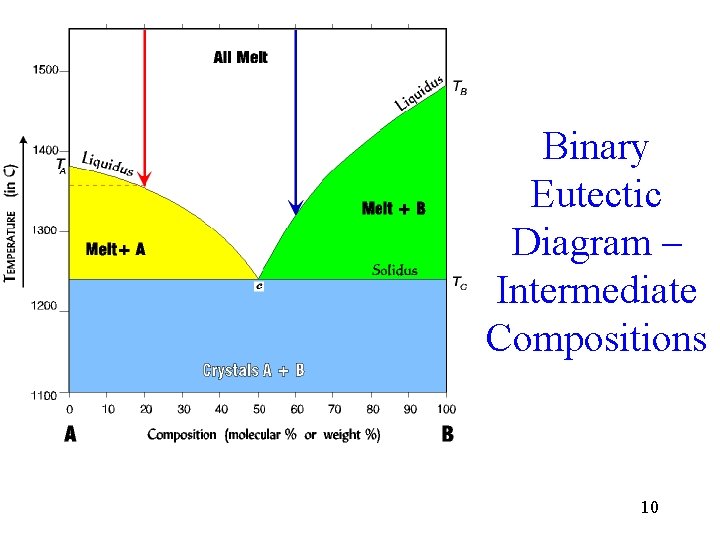
Binary Eutectic Diagram – Intermediate Compositions 10
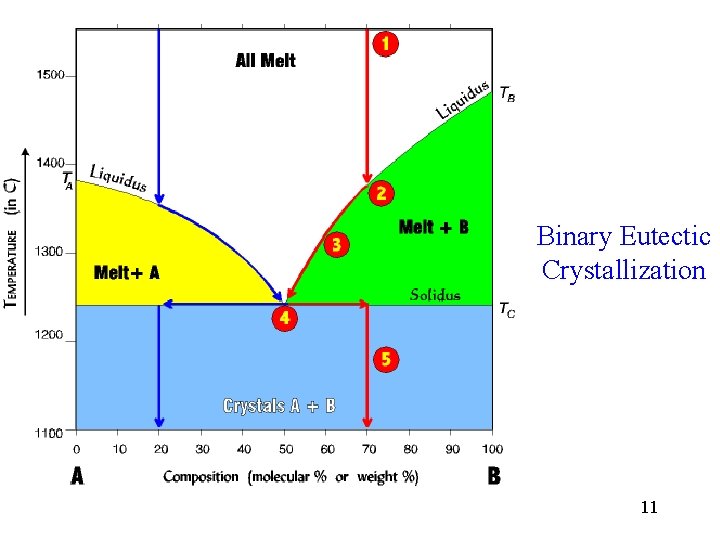
Binary Eutectic Crystallization 11
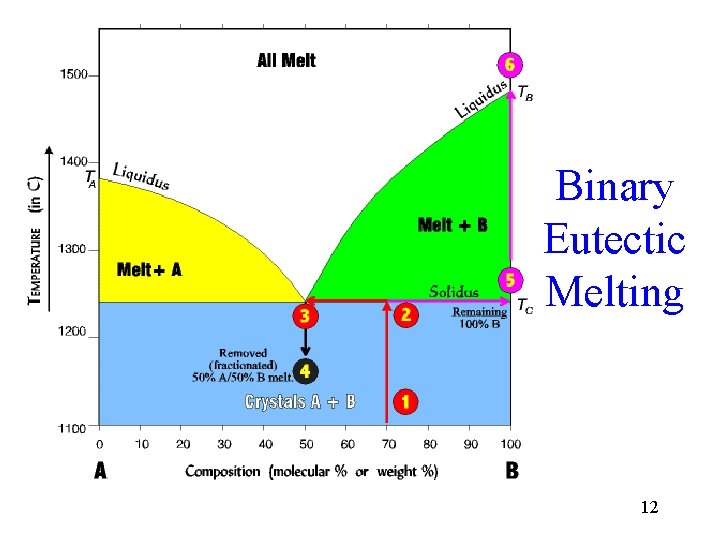
Binary Eutectic Melting 12
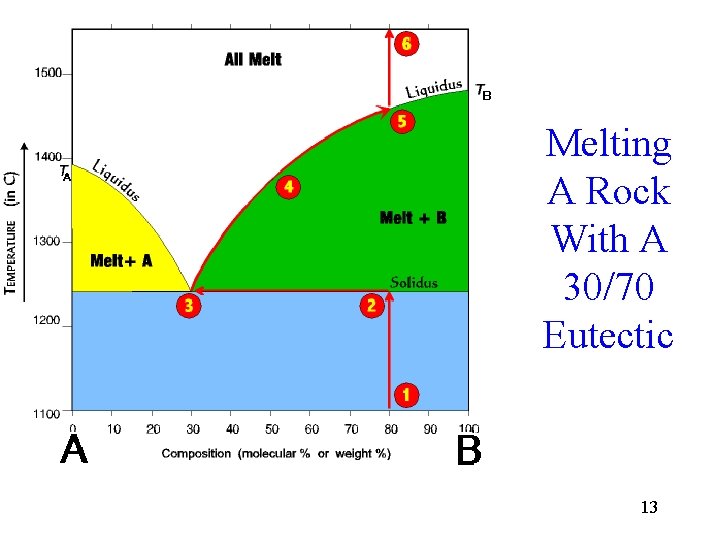
Melting A Rock With A 30/70 Eutectic 13
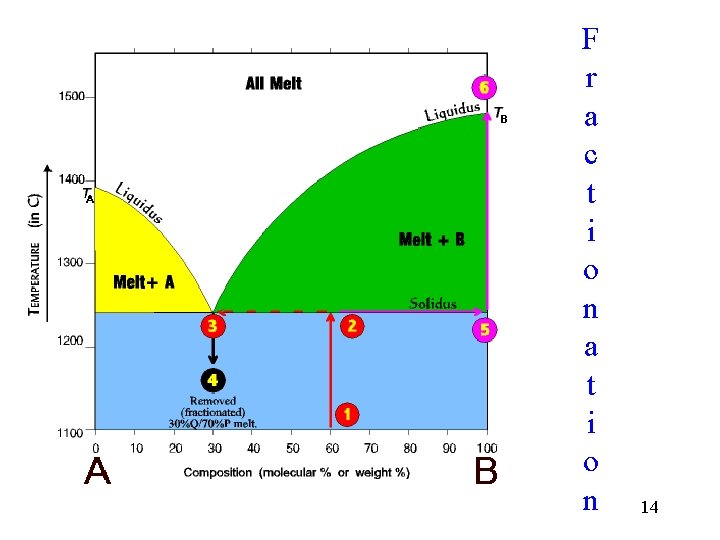
F r a c t i o n a t i o n 14

Congruent Melting • The previous case is an example of congruent melting • Congruent melting means melting of a substance directly to a liquid that is of the same composition as the solid 15

Incongruent Melting • Melting accompanied by decomposition or by reaction with the liquid, so that one solid phase is converted into another • Melting to give a liquid different in composition from the original solid • One example occurs in the forsterite-quartz system 16
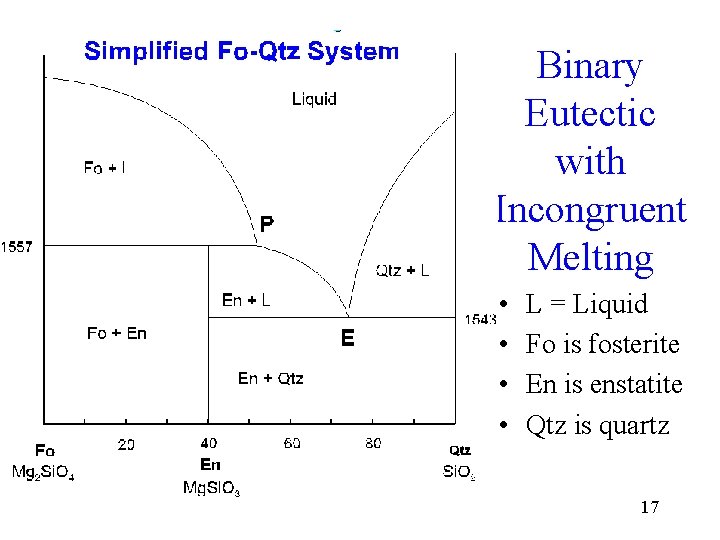
Binary Eutectic with Incongruent Melting • • L = Liquid Fo is fosterite En is enstatite Qtz is quartz 17

Reaction • Mg. Si. O 3 + Si. O 2 = Mg 2 Si. O 4 • En + Qtz = Fo 18
Fo- Qtz 19
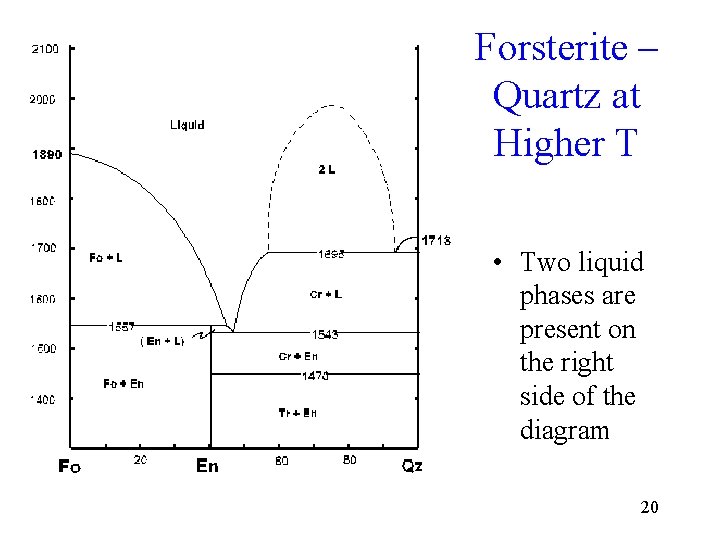
Forsterite – Quartz at Higher T • Two liquid phases are present on the right side of the diagram 20
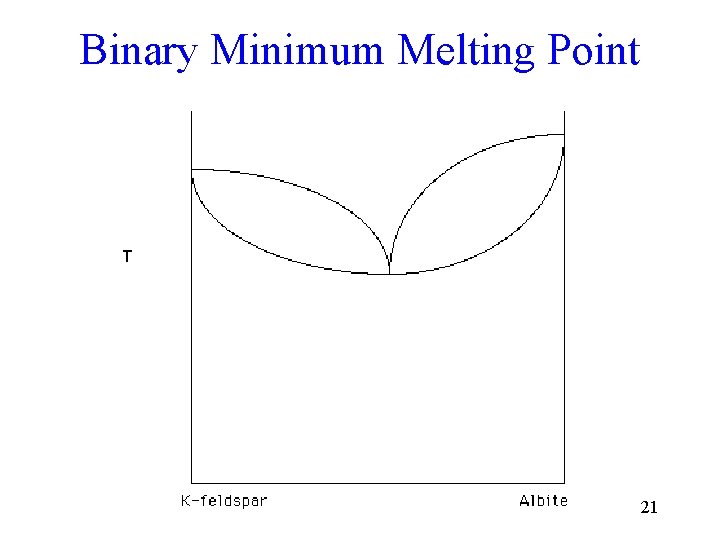
Binary Minimum Melting Point 21
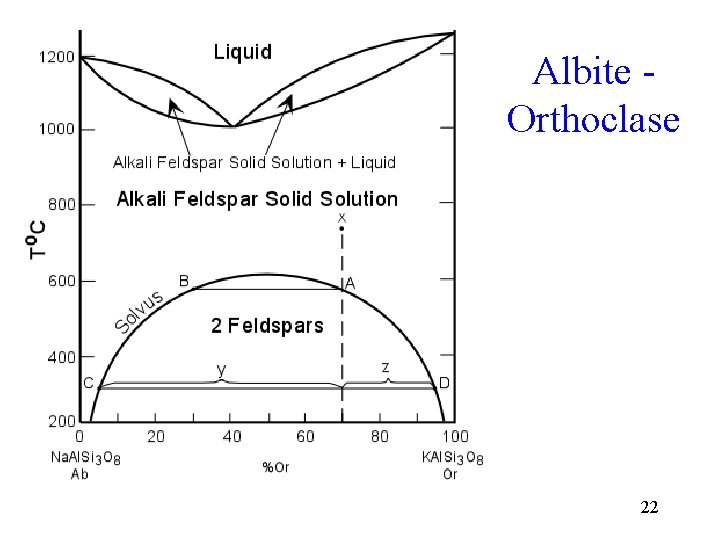
Albite Orthoclase 22
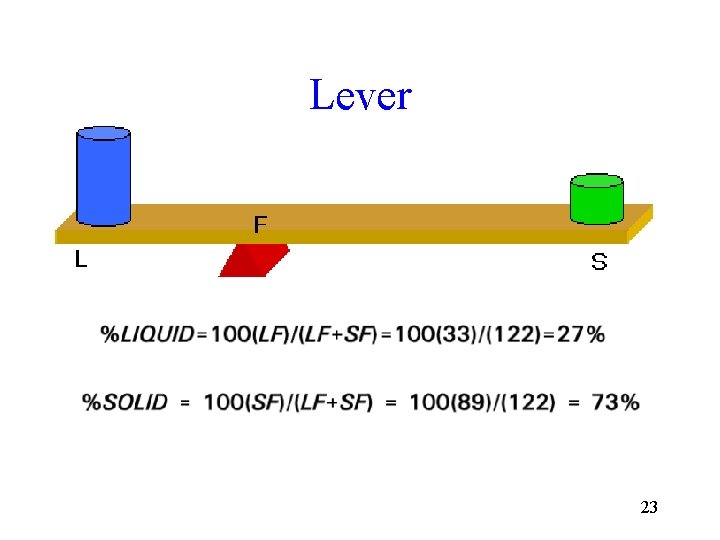
Lever 23
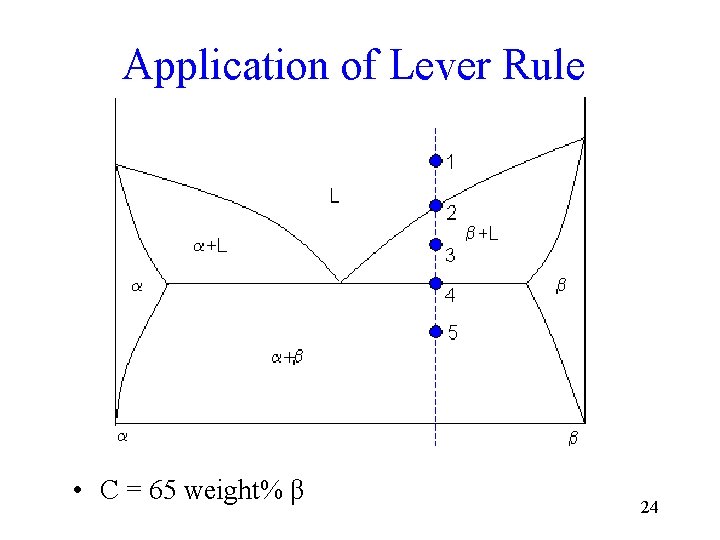
Application of Lever Rule • C = 65 weight% β 24
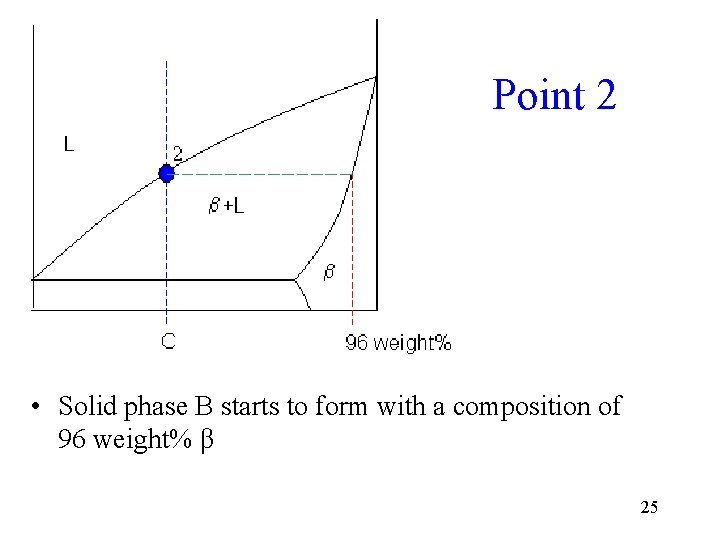
Point 2 • Solid phase B starts to form with a composition of 96 weight% β 25
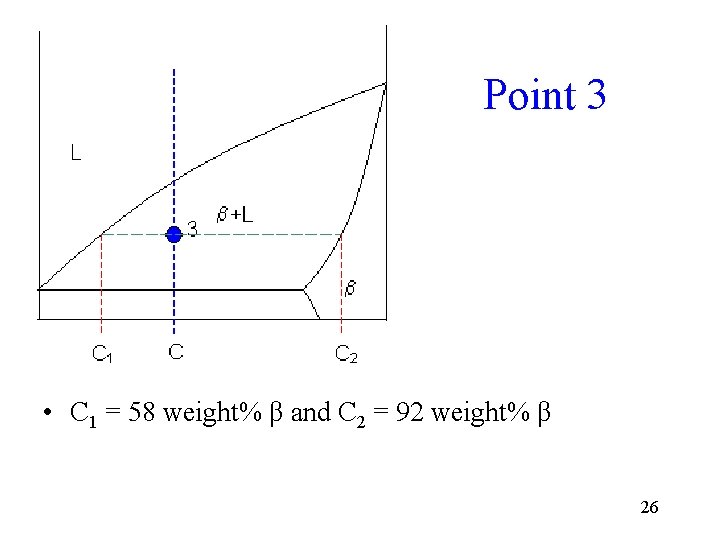
Point 3 • C 1 = 58 weight% β and C 2 = 92 weight% β 26

Point 3 Calculations • Fraction of solid b = (65 - 58) / (92 - 58) = 20 weight% • Fraction of liquid = (92 - 65) / (92 - 58) = 80 weight% 27
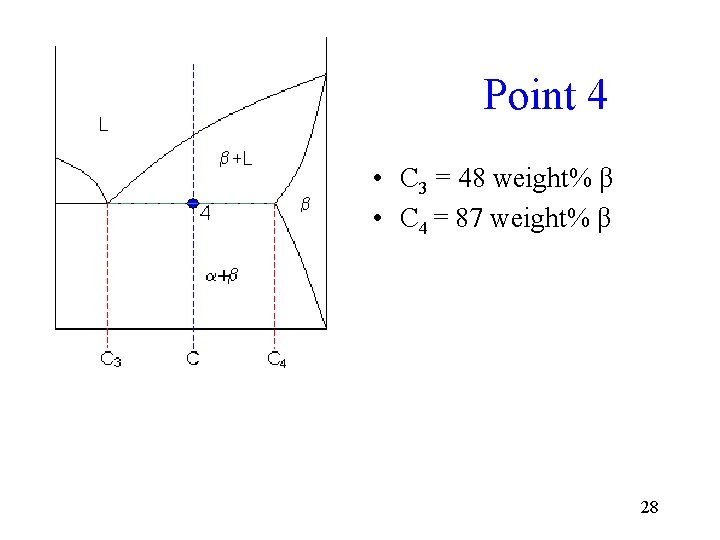
Point 4 • C 3 = 48 weight% β • C 4 = 87 weight% β 28
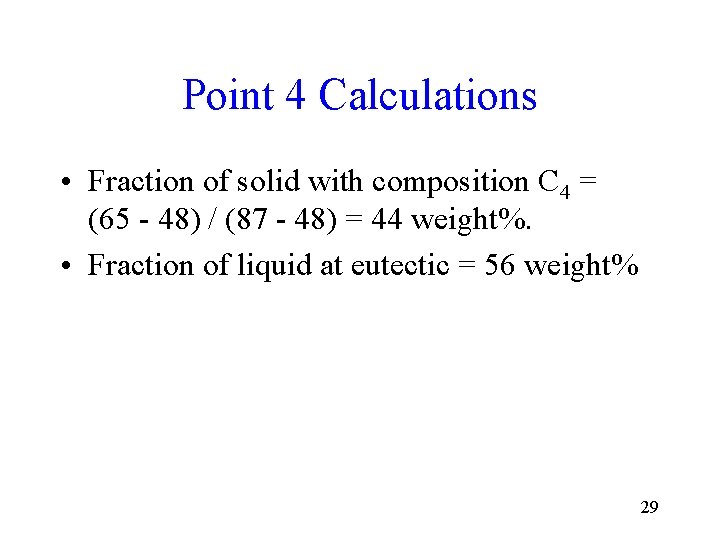
Point 4 Calculations • Fraction of solid with composition C 4 = (65 - 48) / (87 - 48) = 44 weight%. • Fraction of liquid at eutectic = 56 weight% 29
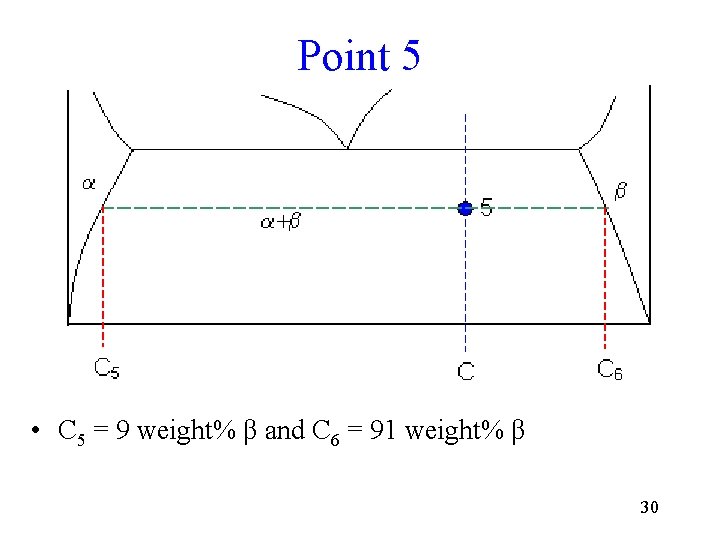
Point 5 • C 5 = 9 weight% β and C 6 = 91 weight% β 30
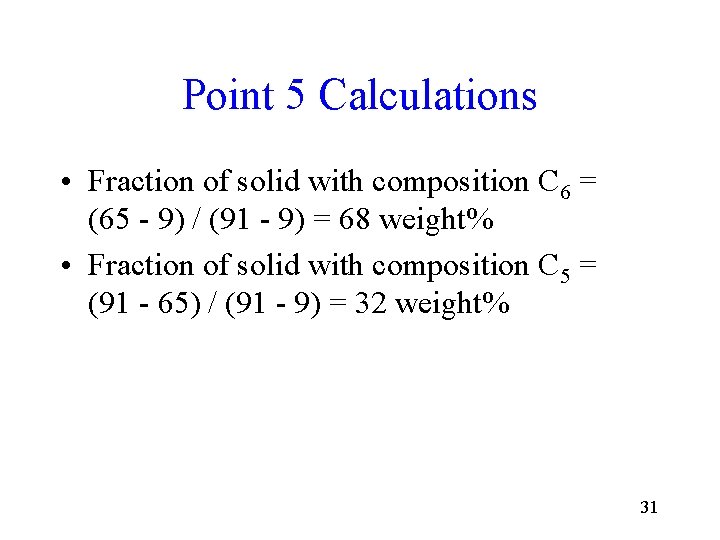
Point 5 Calculations • Fraction of solid with composition C 6 = (65 - 9) / (91 - 9) = 68 weight% • Fraction of solid with composition C 5 = (91 - 65) / (91 - 9) = 32 weight% 31
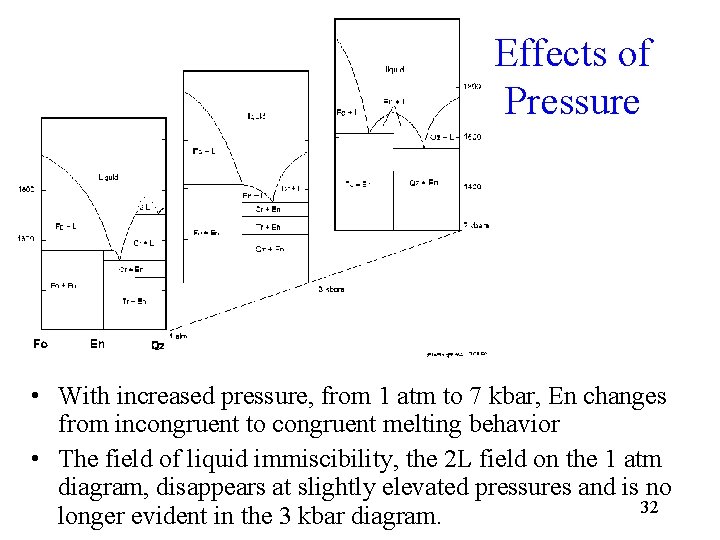
Effects of Pressure • With increased pressure, from 1 atm to 7 kbar, En changes from incongruent to congruent melting behavior • The field of liquid immiscibility, the 2 L field on the 1 atm diagram, disappears at slightly elevated pressures and is no 32 longer evident in the 3 kbar diagram.
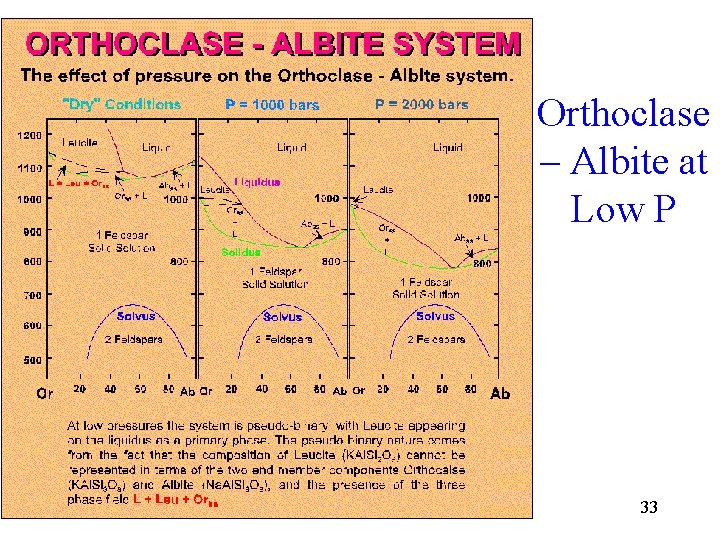
Orthoclase – Albite at Low P 33
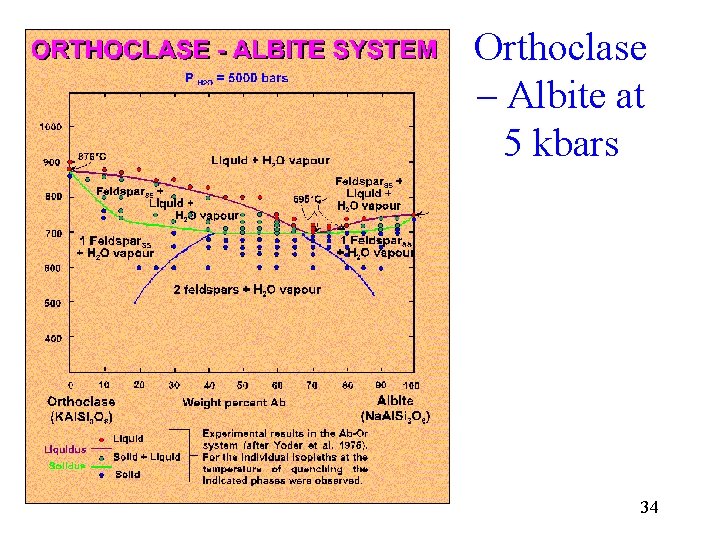
Orthoclase – Albite at 5 kbars 34
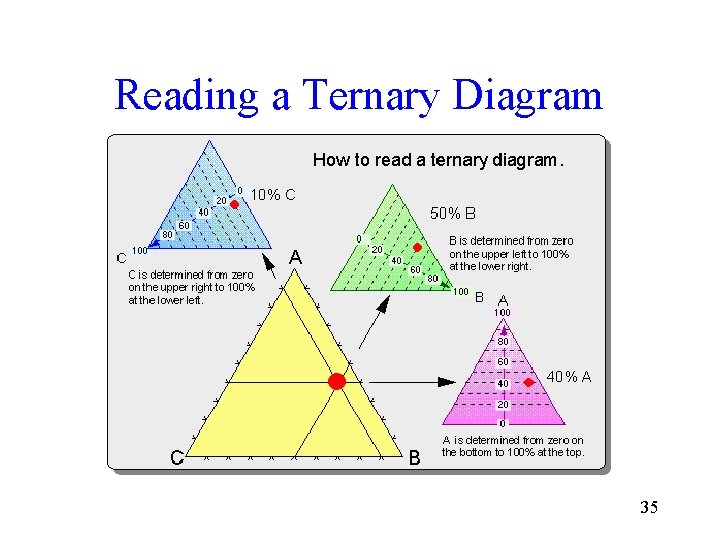
Reading a Ternary Diagram 35
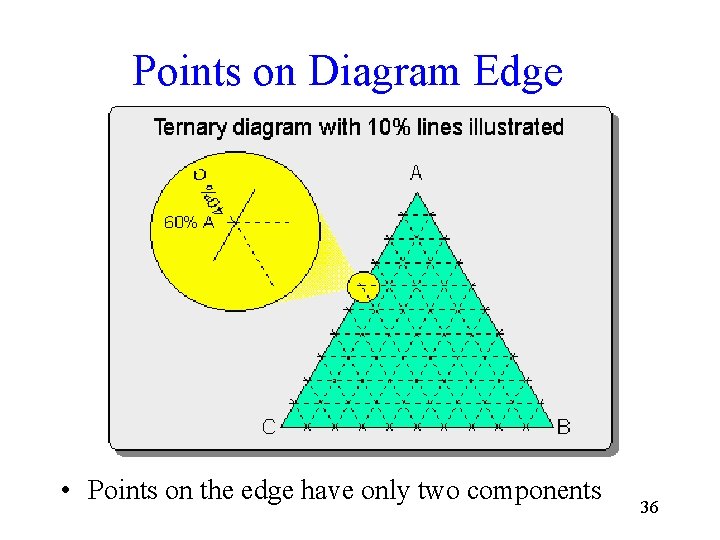
Points on Diagram Edge • Points on the edge have only two components 36
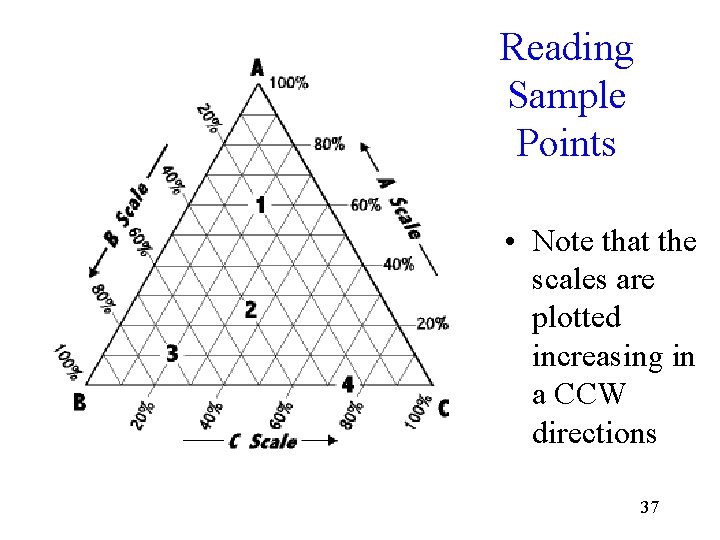
Reading Sample Points • Note that the scales are plotted increasing in a CCW directions 37
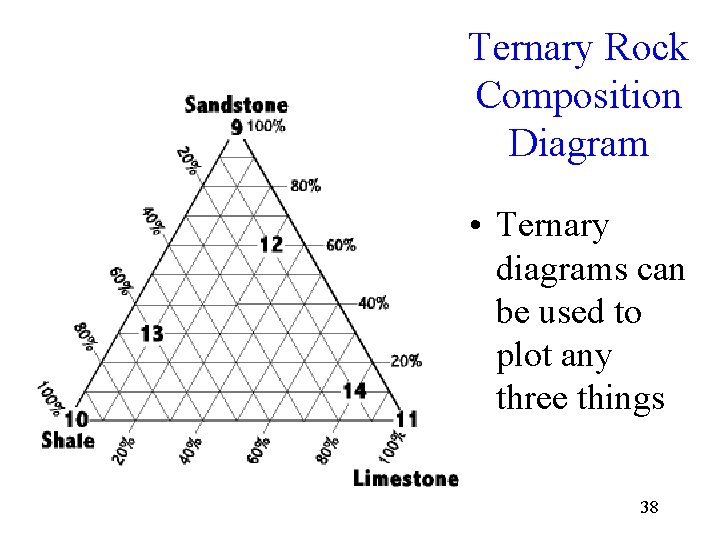
Ternary Rock Composition Diagram • Ternary diagrams can be used to plot any three things 38
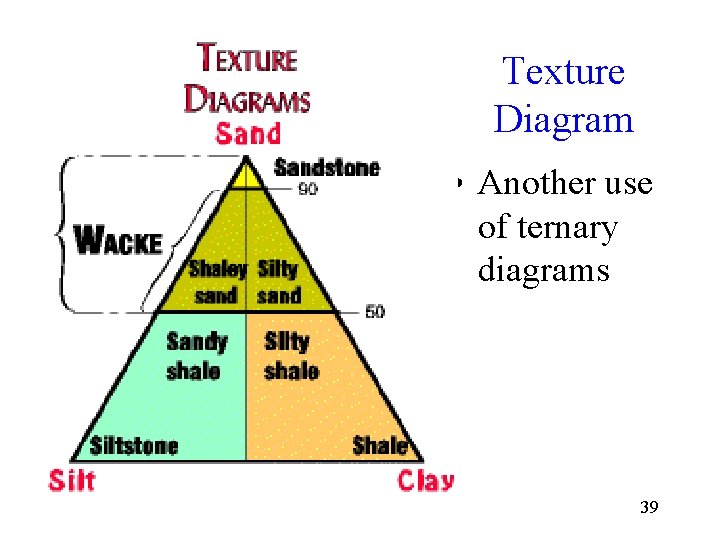
Texture Diagram • Another use of ternary diagrams 39
Ternary System • Sample ternary diagram 40
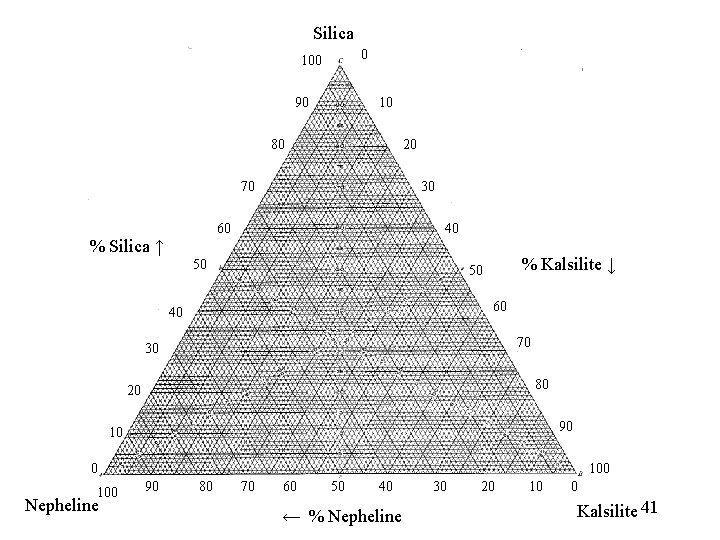
Silica 0 100 90 10 80 20 70 30 60 % Silica ↑ 40 50 % Kalsilite ↓ 50 60 40 70 30 80 20 90 100 Nepheline 100 90 80 70 60 50 40 ← % Nepheline 30 20 10 0 Kalsilite 41
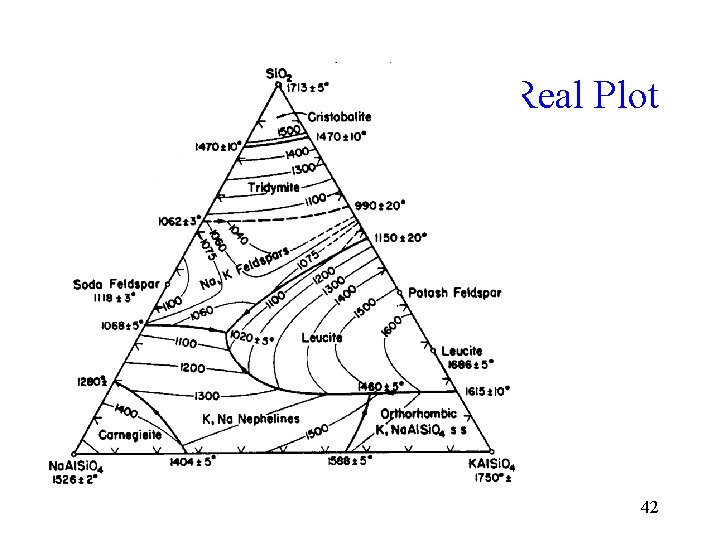
Real Plot 42
- Slides: 42