Binary Phase Diagrams Application to the crystallization of

Binary Phase Diagrams Application to the crystallization of magmas
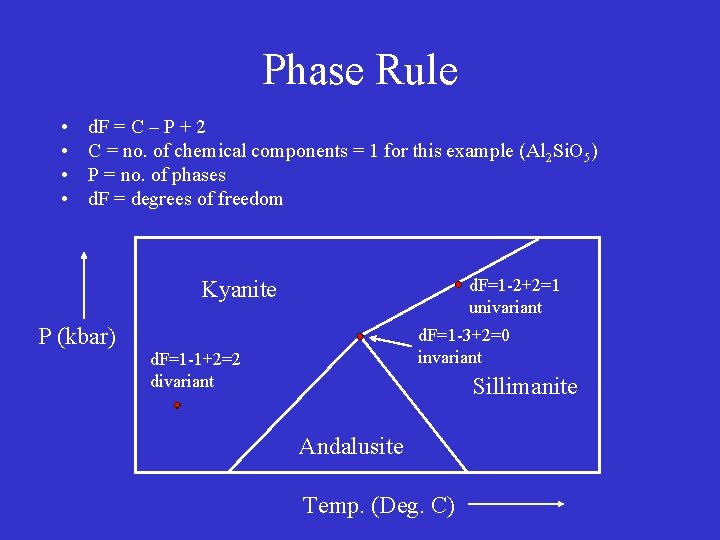
Phase Rule • • d. F = C – P + 2 C = no. of chemical components = 1 for this example (Al 2 Si. O 5) P = no. of phases d. F = degrees of freedom d. F=1 -2+2=1 univariant Kyanite P (kbar) d. F=1 -3+2=0 invariant d. F=1 -1+2=2 divariant Sillimanite Andalusite Temp. (Deg. C)
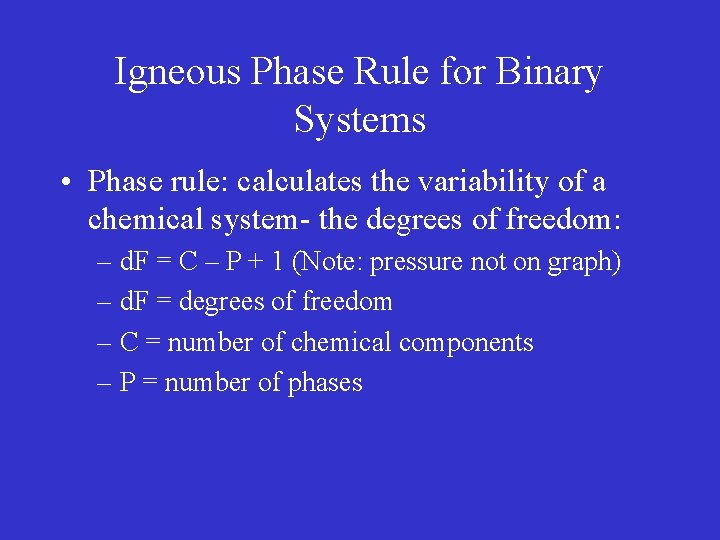
Igneous Phase Rule for Binary Systems • Phase rule: calculates the variability of a chemical system- the degrees of freedom: – d. F = C – P + 1 (Note: pressure not on graph) – d. F = degrees of freedom – C = number of chemical components – P = number of phases
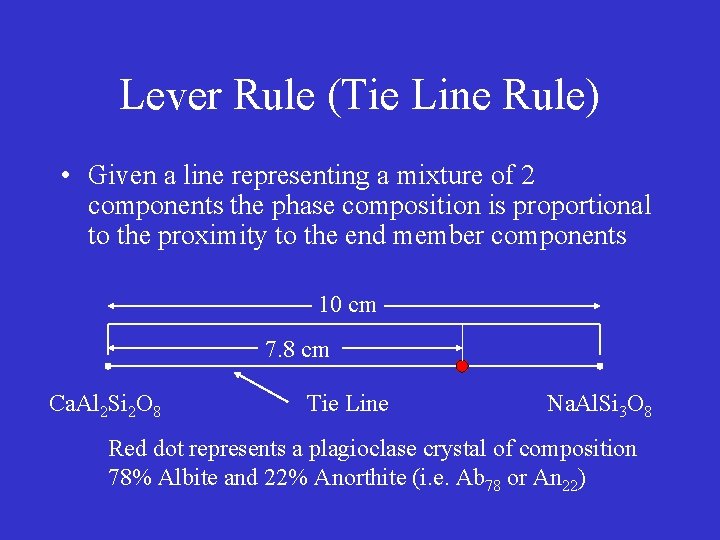
Lever Rule (Tie Line Rule) • Given a line representing a mixture of 2 components the phase composition is proportional to the proximity to the end member components 10 cm 7. 8 cm Ca. Al 2 Si 2 O 8 Tie Line Na. Al. Si 3 O 8 Red dot represents a plagioclase crystal of composition 78% Albite and 22% Anorthite (i. e. Ab 78 or An 22)
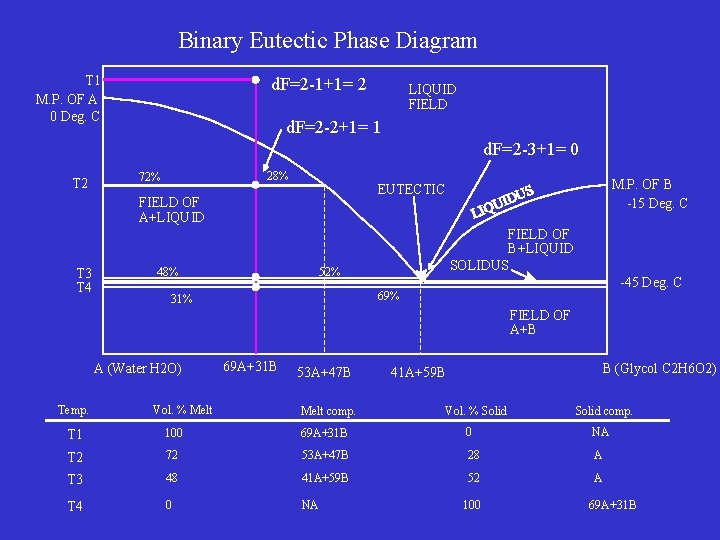
Binary Eutectic Phase Diagram T 1 M. P. OF A 0 Deg. C d. F=2 -1+1= 2 LIQUID FIELD d. F=2 -2+1= 1 d. F=2 -3+1= 0 T 2 28% 72% FIELD OF A+LIQUID T 3 T 4 M. P. OF B -15 Deg. C EUTECTIC 48% FIELD OF B+LIQUID SOLIDUS 52% -45 Deg. C 69% 31% FIELD OF A+B A (Water H 2 O) Temp. Vol. % Melt 69 A+31 B 53 A+47 B Melt comp. B (Glycol C 2 H 6 O 2) 41 A+59 B Vol. % Solid comp. T 1 100 69 A+31 B 0 NA T 2 72 53 A+47 B 28 A T 3 48 41 A+59 B 52 A T 4 0 NA 100 69 A+31 B
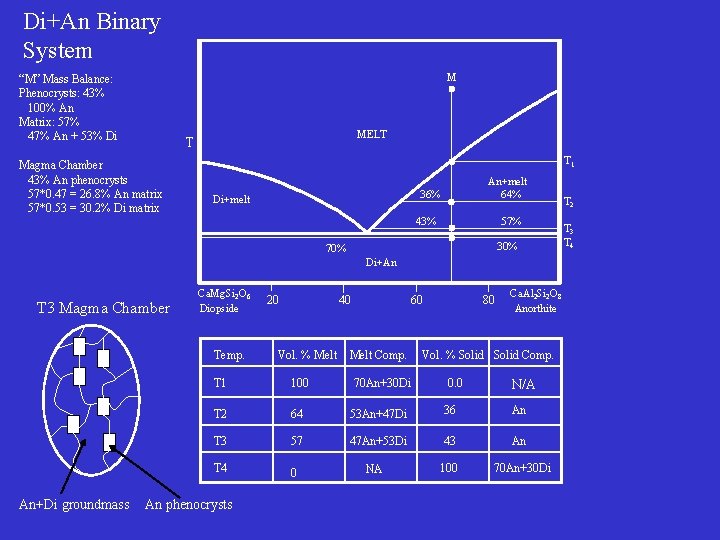
Di+An Binary System M “M” Mass Balance: Phenocrysts: 43% 100% An Matrix: 57% 47% An + 53% Di MELT T Magma Chamber 43% An phenocrysts 57*0. 47 = 26. 8% An matrix 57*0. 53 = 30. 2% Di matrix T 1 An+melt 64% 36% Di+melt 43% 57% 30% 70% Di+An T 3 Magma Chamber Ca. Mg. Si 2 O 6 Diopside Temp. An+Di groundmass 20 40 Vol. % Melt 60 Melt Comp. Ca. Al 2 Si 2 O 8 Anorthite Vol. % Solid Comp. T 1 100 T 2 64 53 An+47 Di 36 An T 3 57 47 An+53 Di 43 An T 4 0 100 70 An+30 Di An phenocrysts 70 An+30 Di 80 NA 0. 0 N/A T 2 T 3 T 4
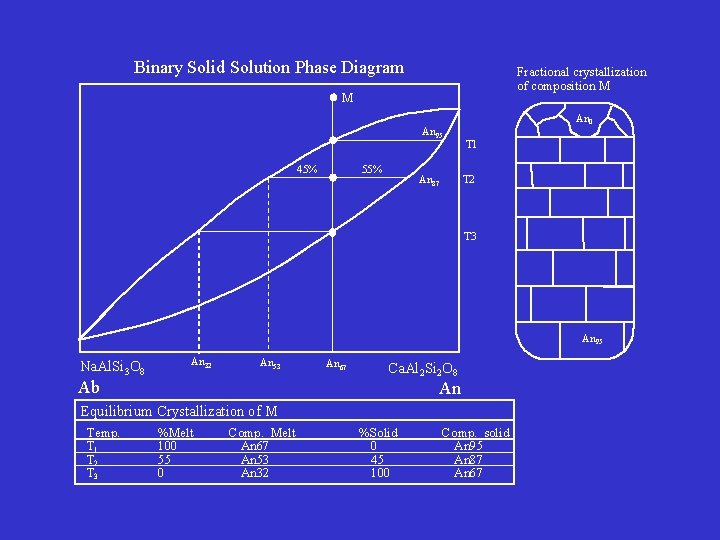
Binary Solid Solution Phase Diagram Fractional crystallization of composition M M An 95 45% 55% An 0 T 1 T 2 An 87 T 3 An 95 Na. Al. Si 3 O 8 An 32 An 53 Ab An 67 Ca. Al 2 Si 2 O 8 An Equilibrium Crystallization of M Temp. T 1 T 2 T 3 %Melt 100 55 0 Comp. Melt An 67 An 53 An 32 %Solid 0 45 100 Comp. solid An 95 An 87 An 67
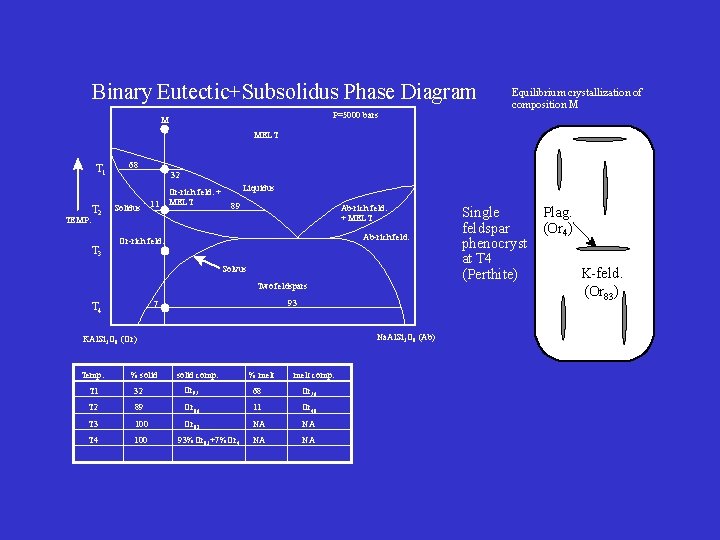
Binary Eutectic+Subsolidus Phase Diagram Equilibrium crystallization of composition M P=5000 bars M MELT T 1 TEMP. T 2 T 3 68 Solidus 32 11 Or-rich feld. + MELT Liquidus 89 Ab-rich feld. + MELT Ab-rich feld. Or-rich feld. Solvus Two feldspars 93 7 T 4 Na. Al. Si 3 O 8 (Ab) KAl. Si 3 O 8 (Or) Temp. % solid comp. % melt comp. T 1 32 Or 97 68 Or 76 T 2 89 Or 86 11 Or 48 T 3 100 Or 82 NA NA T 4 100 NA NA 93%Or 83+7%Or 4 Single Plag. feldspar (Or 4) phenocryst at T 4 K-feld. (Perthite) (Or 83)
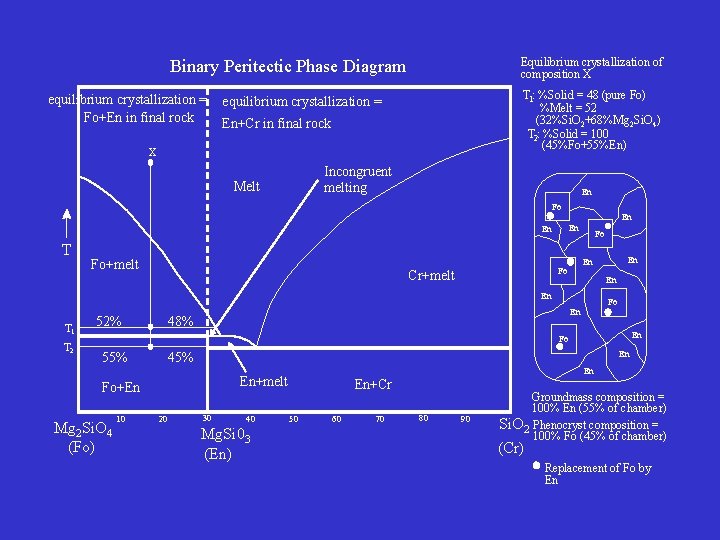
Binary Peritectic Phase Diagram equilibrium crystallization = Fo+En in final rock Equilibrium crystallization of composition X T 1: %Solid = 48 (pure Fo) %Melt = 52 (32%Si. O 2+68%Mg 2 Si. O 4) T 2: %Solid = 100 (45%Fo+55%En) equilibrium crystallization = En+Cr in final rock X Incongruent melting Melt En Fo En En En T Fo+melt En En T 1 T 2 52% Fo En 48% En En Fo Cr+melt Fo En Fo 55% En 45% Fo+En Mg 2 Si. O 4 (Fo) 10 En En+melt 20 30 40 Mg. Si 03 (En) En+Cr 50 60 70 80 90 Groundmass composition = 100% En (55% of chamber) Si. O 2 Phenocryst composition = 100% Fo (45% of chamber) (Cr) Replacement of Fo by En
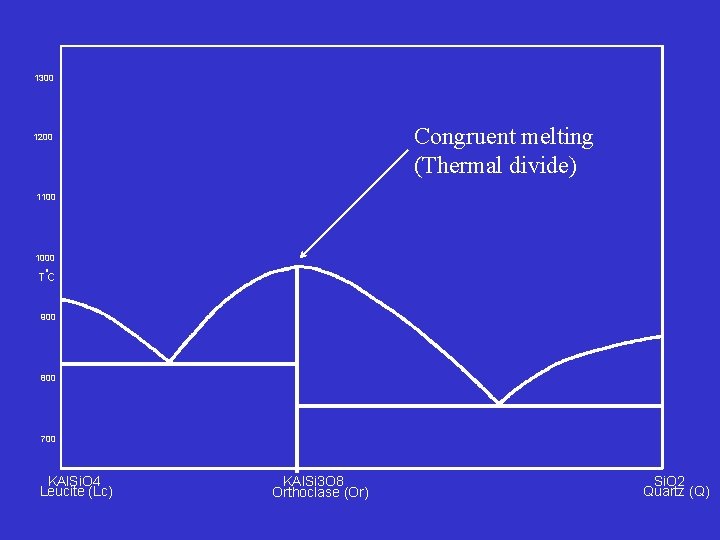
1300 Congruent melting (Thermal divide) 1200 1100 1000 o TC 900 800 700 KAl. Si. O 4 Leucite (Lc) KAl. Si 3 O 8 Orthoclase (Or) Si. O 2 Quartz (Q)

Summary • • Invariant Points: MP, Eutectic, Peritectic Univariant lines: Liquidus, Solidus Divariant fields: Melt, Melt + Solid, Solid Incongruent vs. Congruent melting
- Slides: 11