Binary Molecular Covalent Compounds Molecule a neutral group
Binary Molecular (Covalent) Compounds
• Molecule: a neutral group of elements joined by a covalent compound • Diatomic Molecule: A molecule consisting of two atoms • Diatomic Elements: (7 of them, form a 7 on the PT) and they end in –ine or gen (N, O, F, Cl, Br, I, H)
• Molecular compound: Compound composed of molecules 1. Binary molecular compounds are made up of 2 elements 2. Typically these elements are nonmetals 3. There are no charges in a molecular compound 4. Molecular compounds are held together by covalent bonds
5. A covalent bond is a sharing of electrons so that each element forms an octet 6. We can show the sharing of e- by drawing Lewis structures (dot diagrams) 7. The first rule for drawing a molecular Lewis structure is to get the formula

8. Next, count the number of electrons 9. Each bond that is formed requires 2 electrons 10. We can show a single bond with 2 electrons or with 1 line 11. We can show a double bond with 4 e- or with 2 lines 12. We can show a triple bond with 6 e- or with three lines

13. Valence electrons that are shared b/w 2 elements are called bonding electrons 14. Valence e- that are not shared b/w 2 elements are called lone pair electrons 15. In the end, each element should always have/share 8 e-, except for H b/c this element can only have 2 e-


• CO 2 • N 2 • HCN • SO 3

16. A resonance structure is a structure that occurs when two or more valid electron dot structures are able to be drawn. 17. An arrow indicates multiple resonance structures. See page 224 in book

• Examples: • SO 3 • O 3
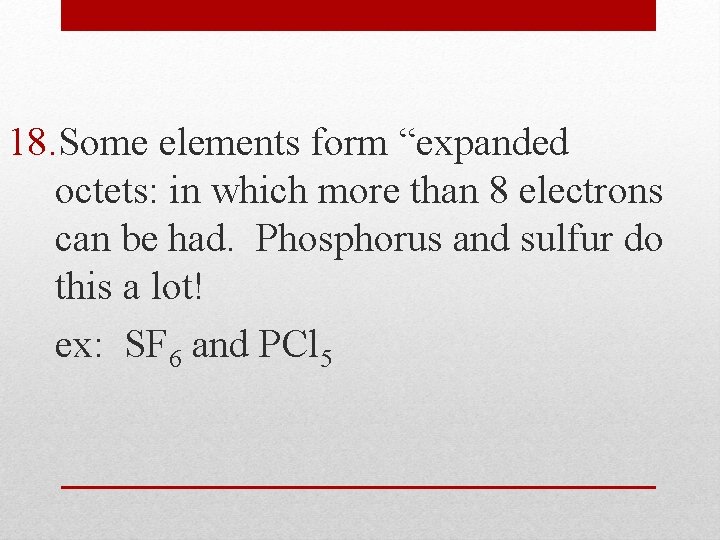
18. Some elements form “expanded octets: in which more than 8 electrons can be had. Phosphorus and sulfur do this a lot! ex: SF 6 and PCl 5
- Slides: 11