Binary Ionic Compounds Nomenclature Write on your PT
Binary Ionic Compounds & Nomenclature
Write on your PT…
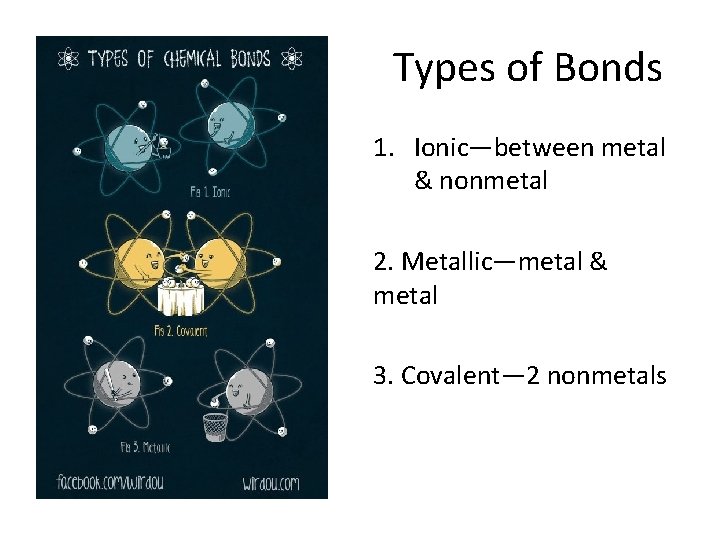
Types of Bonds 1. Ionic—between metal & nonmetal 2. Metallic—metal & metal 3. Covalent— 2 nonmetals

Ionic bond • Non-metal + Metal • Example: Na. CL • Na+, (Sodium is a metal) • Cl-, (Chlorine is a non-metal)
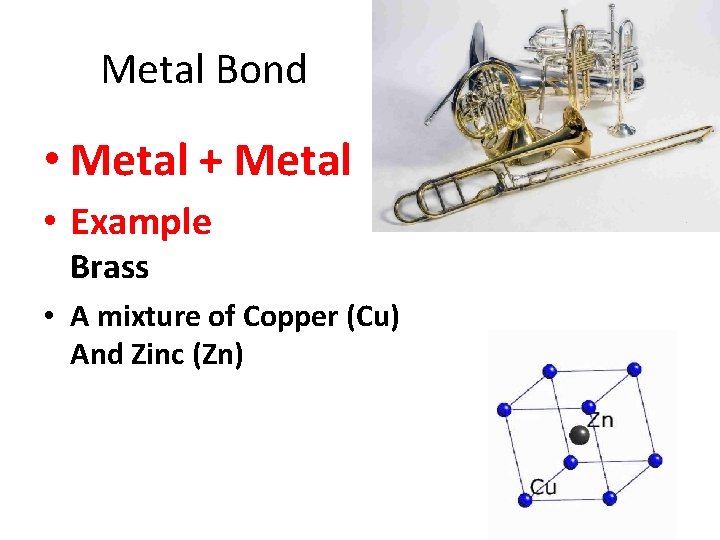
Metal Bond • Metal + Metal • Example Brass • A mixture of Copper (Cu) And Zinc (Zn)
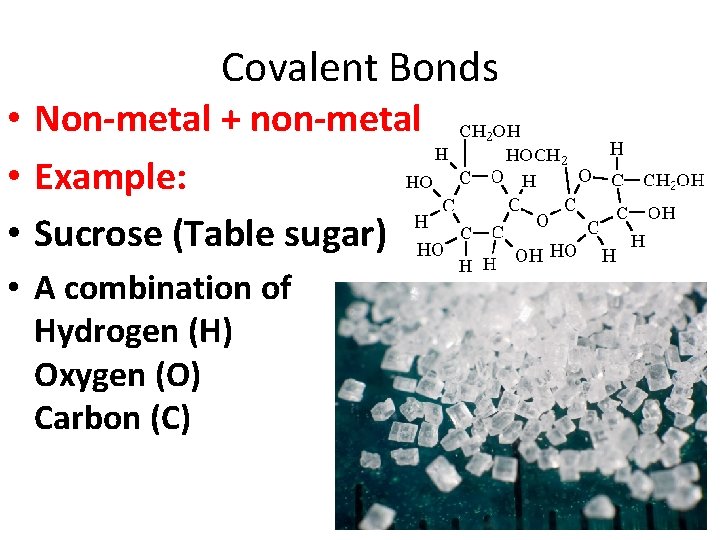
Covalent Bonds • Non-metal + non-metal • Example: • Sucrose (Table sugar) • A combination of Hydrogen (H) Oxygen (O) Carbon (C)
Why atoms form bonds: Octet Rule: Atoms tend to gain, lose, or share electrons until they are surrounded by eight valence electrons. • This is decided by the electronegativity. – A small difference is a covalent bond and shared electrons. – A big difference is an ionic bond, and lost/gained electrons.
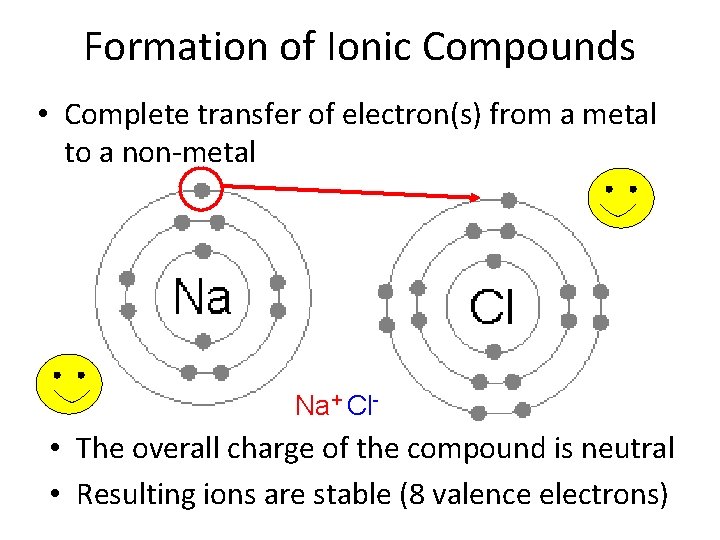
Formation of Ionic Compounds • Complete transfer of electron(s) from a metal to a non-metal Na+ Cl- • The overall charge of the compound is neutral • Resulting ions are stable (8 valence electrons)
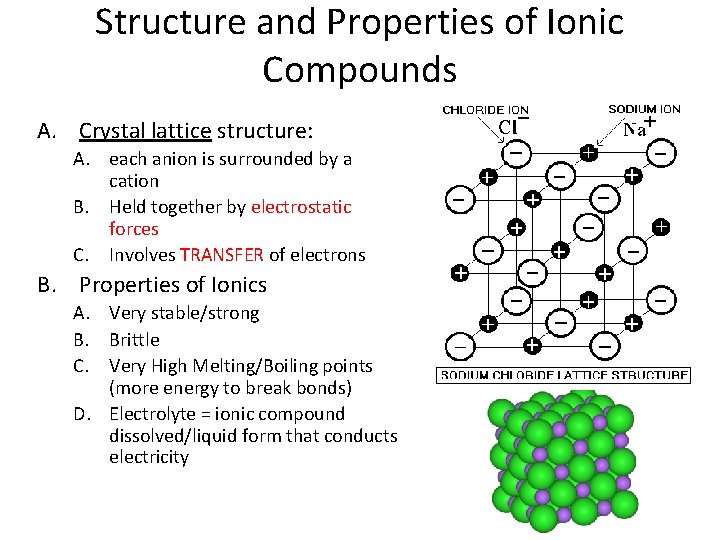
Structure and Properties of Ionic Compounds A. Crystal lattice structure: A. each anion is surrounded by a cation B. Held together by electrostatic forces C. Involves TRANSFER of electrons B. Properties of Ionics A. Very stable/strong B. Brittle C. Very High Melting/Boiling points (more energy to break bonds) D. Electrolyte = ionic compound dissolved/liquid form that conducts electricity
Binary Ionic Compounds • When a metal loses electrons to form a cation and a nonmetal gains electrons to form an anion, the resulting substance is called a binary ionic compound. • There are 3 types: I, II, & III.

Naming Binary Ionics: Type I • Type I compounds: the metal forms only one type of cation • Group 1, 2 & 13 metals are Type 1 • Name using the ions: – Name the cation 1 st and the anion 2 nd – Example: sodium (Na+1) plus chlorine (Cl-1) combine to make sodium chloride *** the anion always ends in –ide
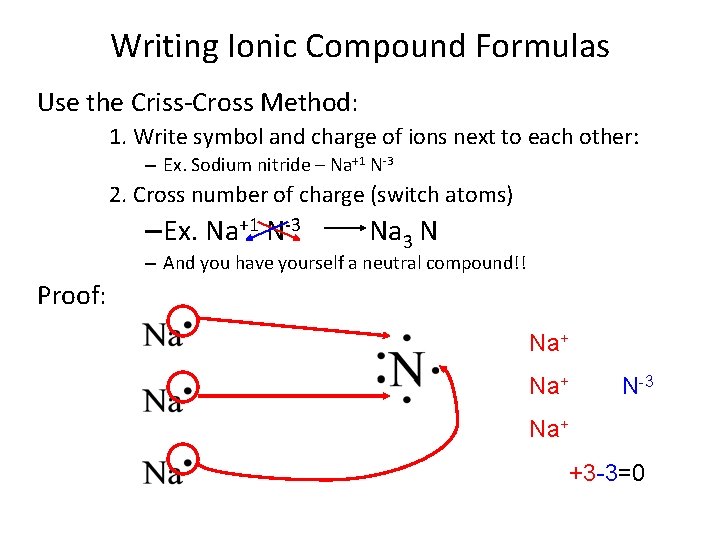
Writing Ionic Compound Formulas Use the Criss-Cross Method: 1. Write symbol and charge of ions next to each other: – Ex. Sodium nitride – Na+1 N-3 2. Cross number of charge (switch atoms) – Ex. Na+1 N-3 Na 3 N – And you have yourself a neutral compound!! Proof: Na+ N-3 Na+ +3 -3=0
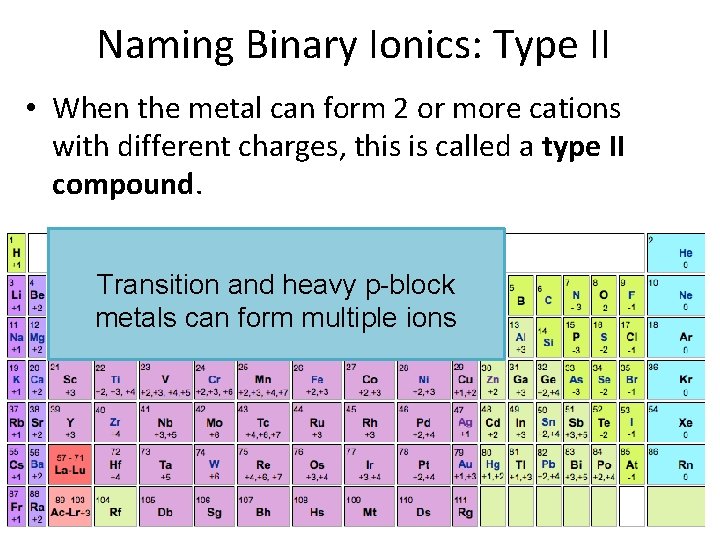
Naming Binary Ionics: Type II • When the metal can form 2 or more cations with different charges, this is called a type II compound. Transition and heavy p-block metals can form multiple ions
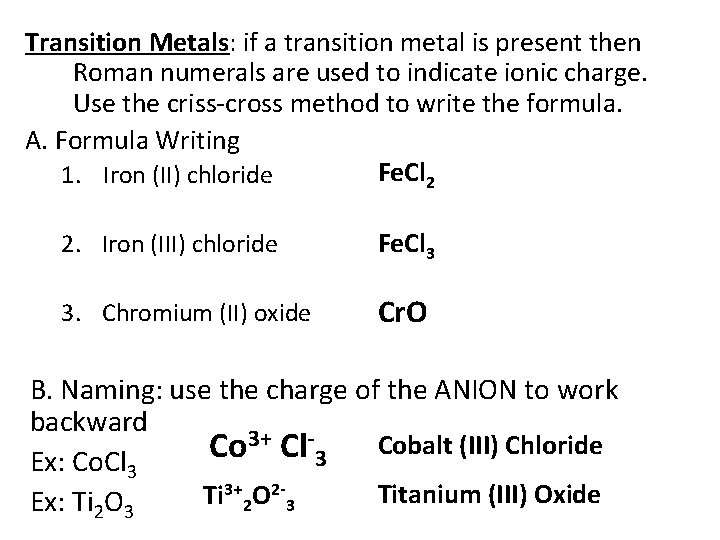
Transition Metals: if a transition metal is present then Roman numerals are used to indicate ionic charge. Use the criss-cross method to write the formula. A. Formula Writing Fe. Cl 2 1. Iron (II) chloride 2. Iron (III) chloride Fe. Cl 3 3. Chromium (II) oxide Cr. O B. Naming: use the charge of the ANION to work backward 3+ Cl. Cobalt (III) Chloride Co 3 Ex: Co. Cl 3 3+ O 2 Titanium (III) Oxide Ti Ex: Ti 2 O 3 2 3
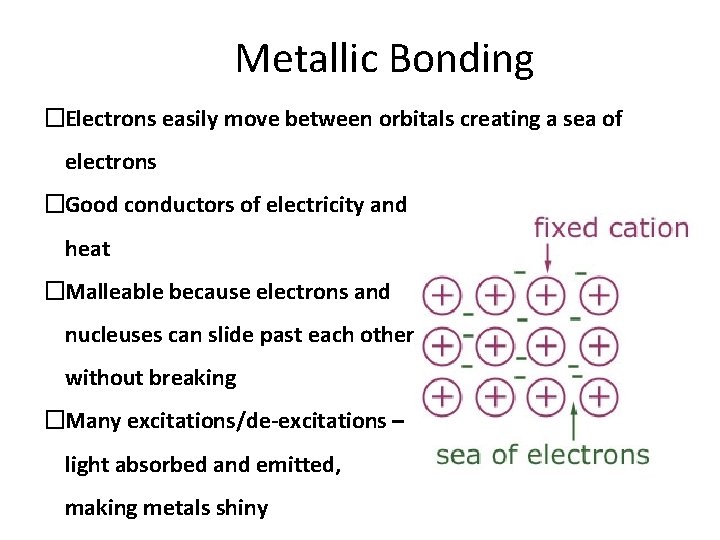
Metallic Bonding �Electrons easily move between orbitals creating a sea of electrons �Good conductors of electricity and heat �Malleable because electrons and nucleuses can slide past each other without breaking �Many excitations/de-excitations – light absorbed and emitted, making metals shiny
Why the penny changes The first change • Na. OH can dissolve the zinc metal, which then precipitates, (Un-dissolves), onto the coin. • The layer of Zinc looks “silver” The second change • When you heat metals, the atoms are free to mix and mingle. • This mix of metals is called an alloy, and will have different properties then either of the starting metals
- Slides: 16