Binary Ionic Compounds Formula to Name The Language

Binary Ionic Compounds Formula to Name
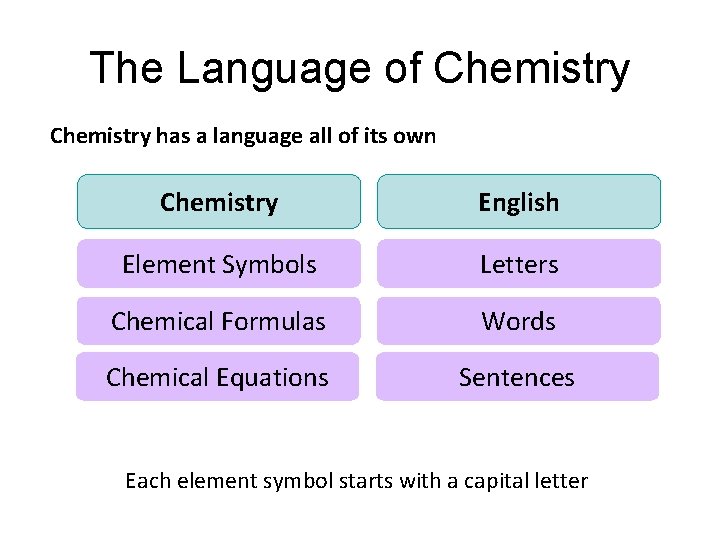
The Language of Chemistry has a language all of its own Chemistry English Element Symbols Letters Chemical Formulas Words Chemical Equations Sentences Each element symbol starts with a capital letter
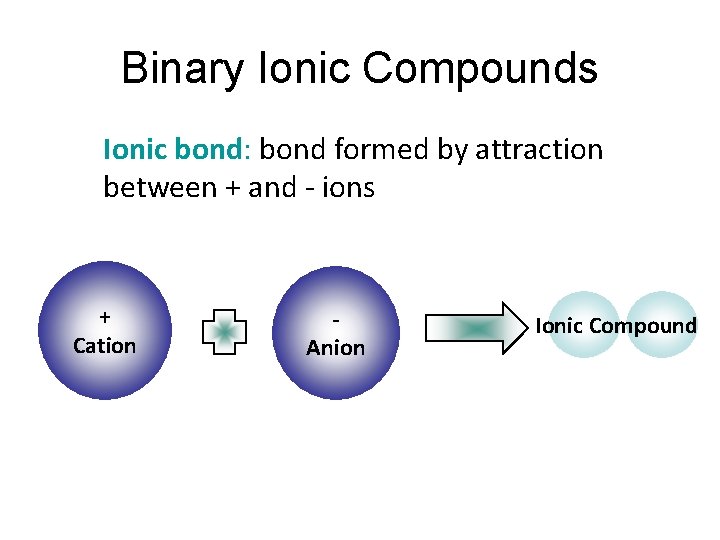
Binary Ionic Compounds Ionic bond: bond formed by attraction between + and - ions + Cation Anion Ionic Compound
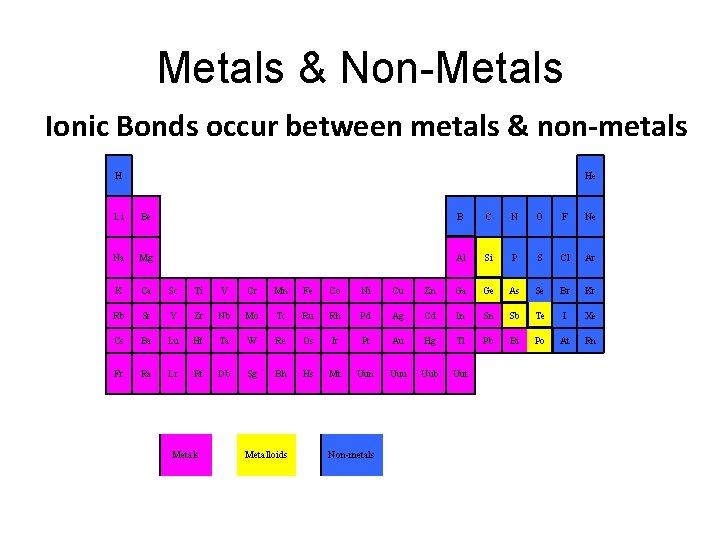
Metals & Non-Metals Ionic Bonds occur between metals & non-metals H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub Uut Metals Metalloids Non-metals
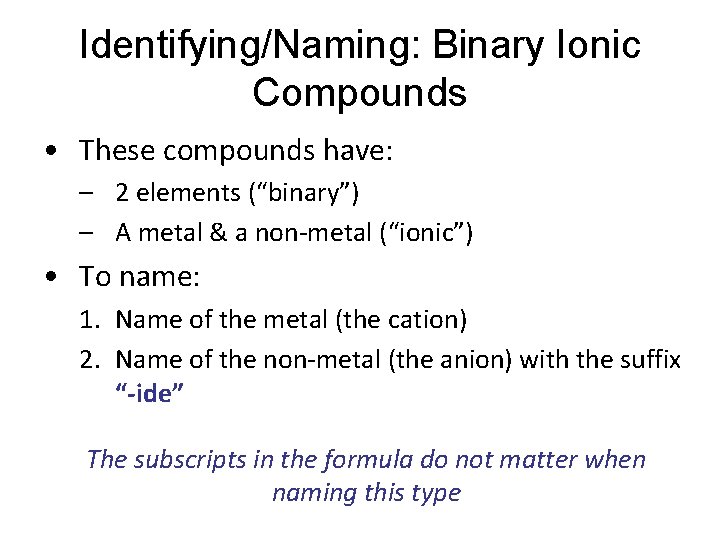
Identifying/Naming: Binary Ionic Compounds • These compounds have: – 2 elements (“binary”) – A metal & a non-metal (“ionic”) • To name: 1. Name of the metal (the cation) 2. Name of the non-metal (the anion) with the suffix “-ide” The subscripts in the formula do not matter when naming this type
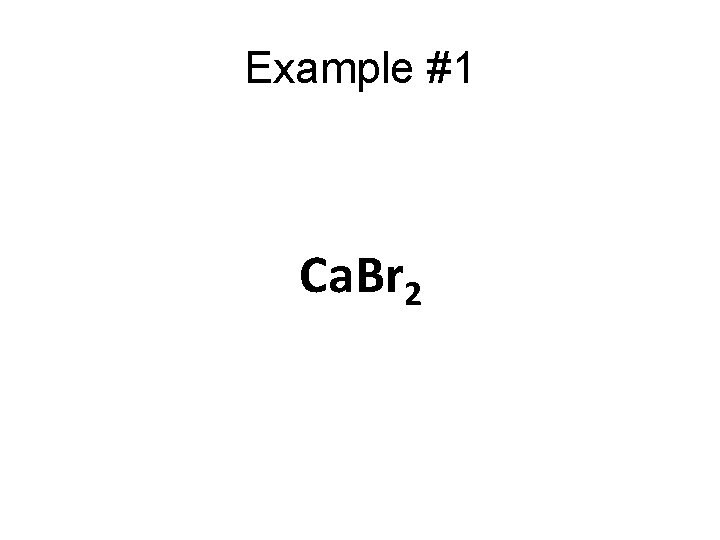
Example #1 Ca. Br 2
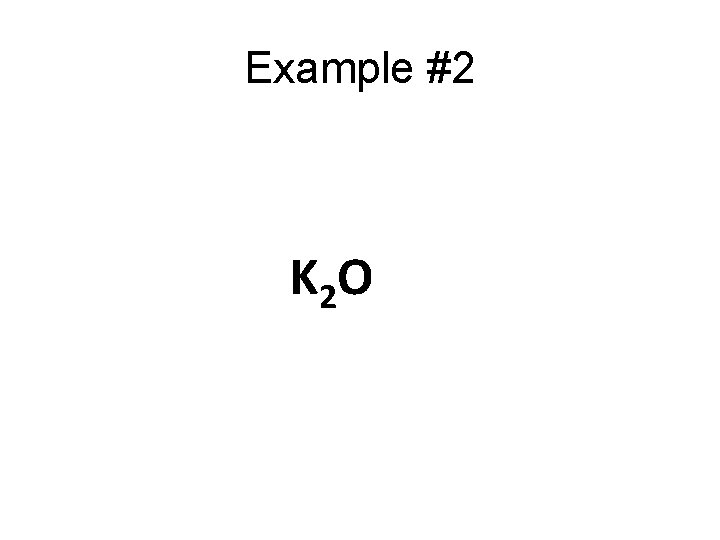
Example #2 K 2 O

Let’s Practice Example: Write the name for the following compounds Ca. F 2 Na 3 P Na. I Sr. Br 2

Let’s Practice Example: Write the name for the following compounds Ca. F 2 Calcium fluoride Na 3 P Sodium phosphide Na. Cl Sodium chloride Sr. Br 2 Strontium bromide

Binary Ionic Compounds Name to Formula

Writing Formulas: Binary Ionic • These compounds: – End in “-ide” (except “hydroxide and cyanide”) – Do NOT contain covalent prefixes • To write: 1. Symbol & charge of the metal cation 2. Symbol & charge of the non-metal anion 3. Add more of the cations and/or anions until you have a neutral compound 4. Use subscripts to show many of each type of ion you have

Example Sodium fluoride
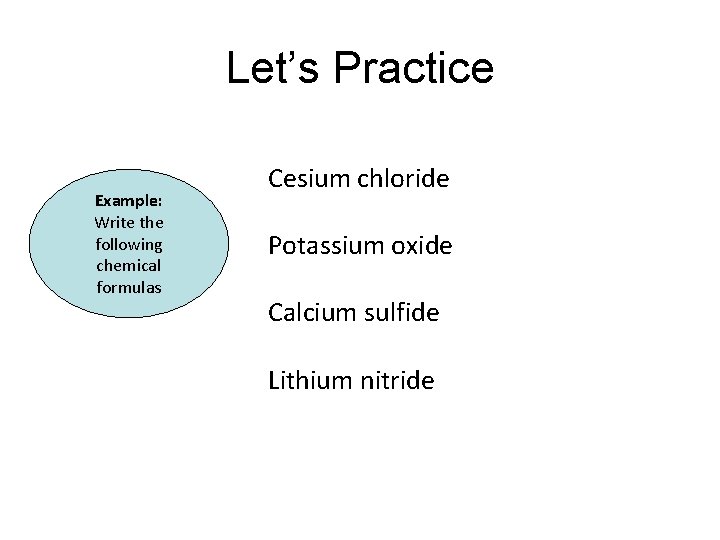
Let’s Practice Example: Write the following chemical formulas Cesium chloride Potassium oxide Calcium sulfide Lithium nitride
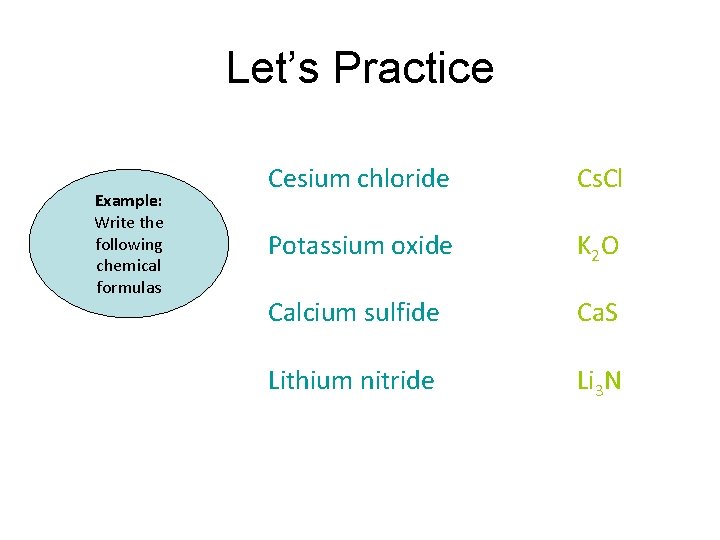
Let’s Practice Example: Write the following chemical formulas Cesium chloride Cs. Cl Potassium oxide K 2 O Calcium sulfide Ca. S Lithium nitride Li 3 N

Multivalent Ionic Compounds Formula to Name
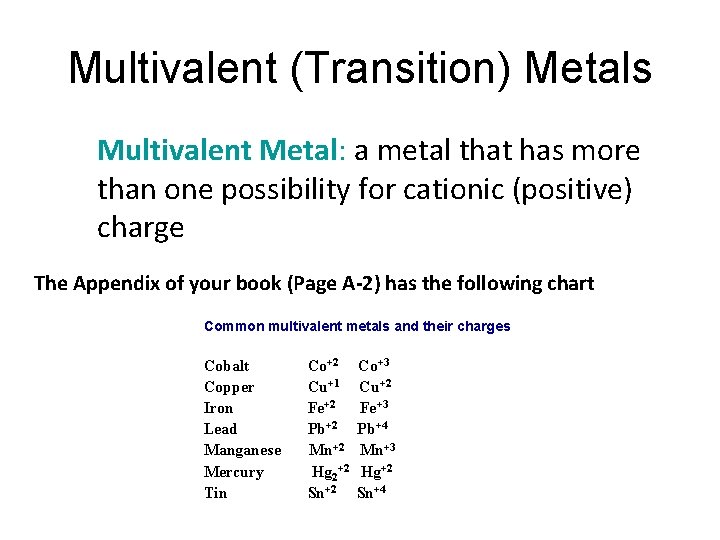
Multivalent (Transition) Metals Multivalent Metal: a metal that has more than one possibility for cationic (positive) charge The Appendix of your book (Page A-2) has the following chart Common multivalent metals and their charges Cobalt Copper Iron Lead Manganese Mercury Tin Co+2 Cu+1 Fe+2 Pb+2 Mn+2 Hg 2+2 Sn+2 Co+3 Cu+2 Fe+3 Pb+4 Mn+3 Hg+2 Sn+4

Multivalent Metals: Formula to Name • To identify: – Have a transition metal • To name these compounds: 1. 2. 3. 4. 5. 6. Name of the metal cation Name of the anion Determine total negative charge Total negative charge must = total positive charge Determine the charge on each metal atom Write the charge in roman numerals in parenthesis after the metal’s name
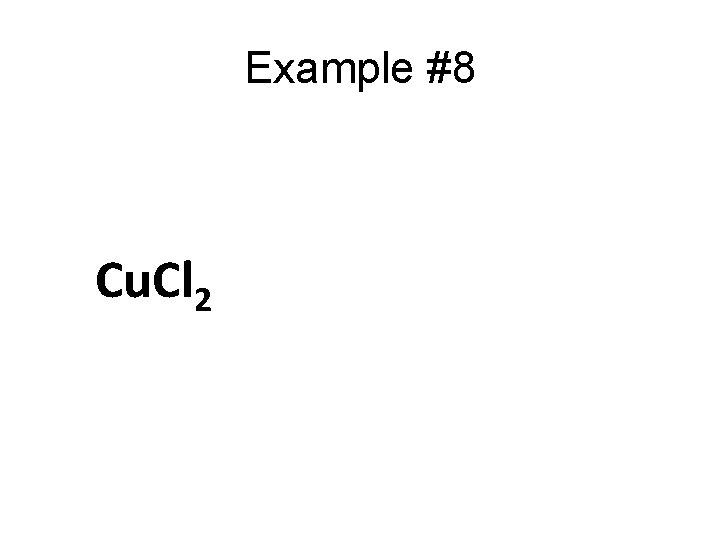
Example #8 Cu. Cl 2
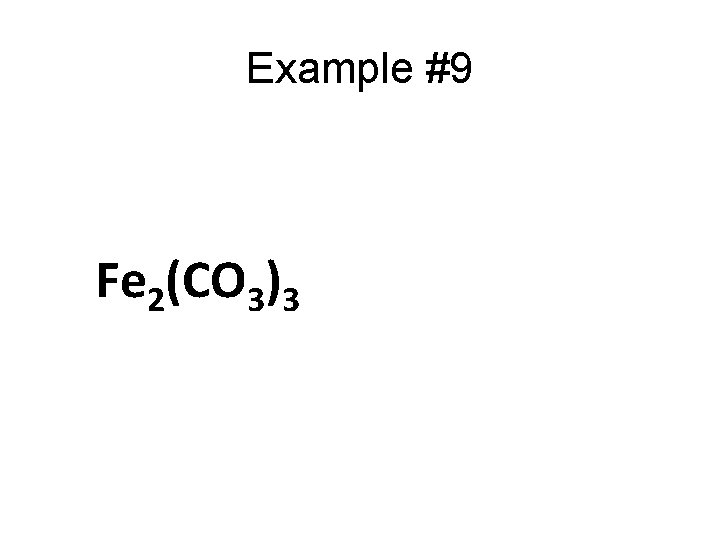
Example #9 Fe 2(CO 3)3
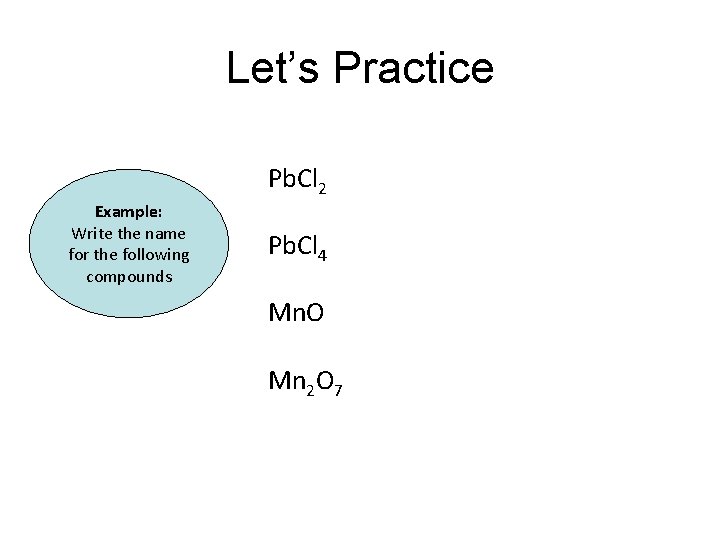
Let’s Practice Pb. Cl 2 Example: Write the name for the following compounds Pb. Cl 4 Mn. O Mn 2 O 7
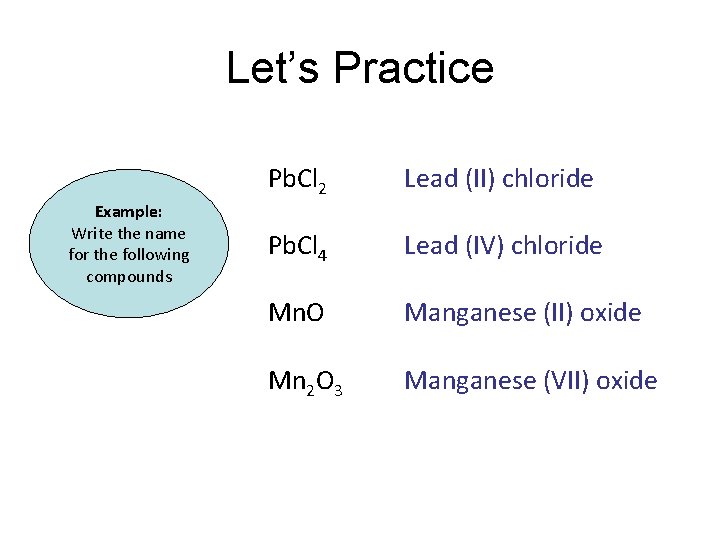
Let’s Practice Example: Write the name for the following compounds Pb. Cl 2 Lead (II) chloride Pb. Cl 4 Lead (IV) chloride Mn. O Manganese (II) oxide Mn 2 O 3 Manganese (VII) oxide

Multivalent Ionic Compounds Name to Formula

Multivalent Metals: Name to Formula • To identify: – Will have roman numerals • To write these formulas: – Same as binary – Roman numerals tell the charge of the metal cation
Example #5 Iron (III) oxide
Example #6 Copper (I) nitride
Let’s Practice Example: Write the following chemical formulas Iron (II) nitride Copper (I) chloride Lead (IV) sulfide Tin (II) oxide
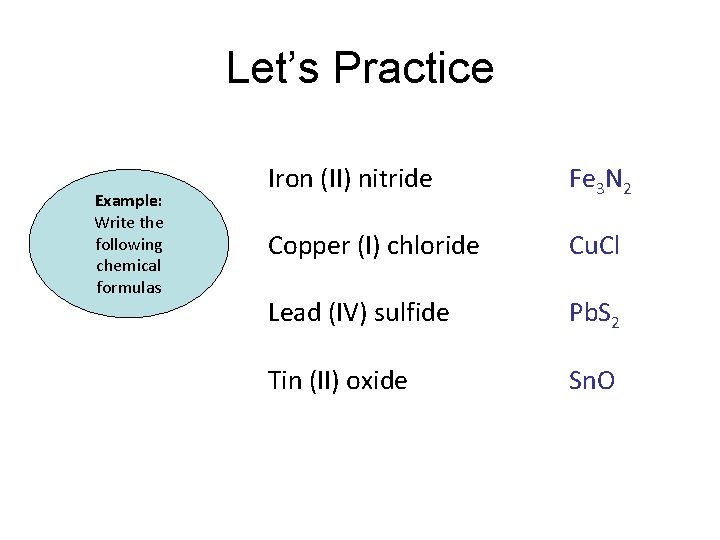
Let’s Practice Example: Write the following chemical formulas Iron (II) nitride Fe 3 N 2 Copper (I) chloride Cu. Cl Lead (IV) sulfide Pb. S 2 Tin (II) oxide Sn. O

Polyatomic Ionic Compounds Formula to Name
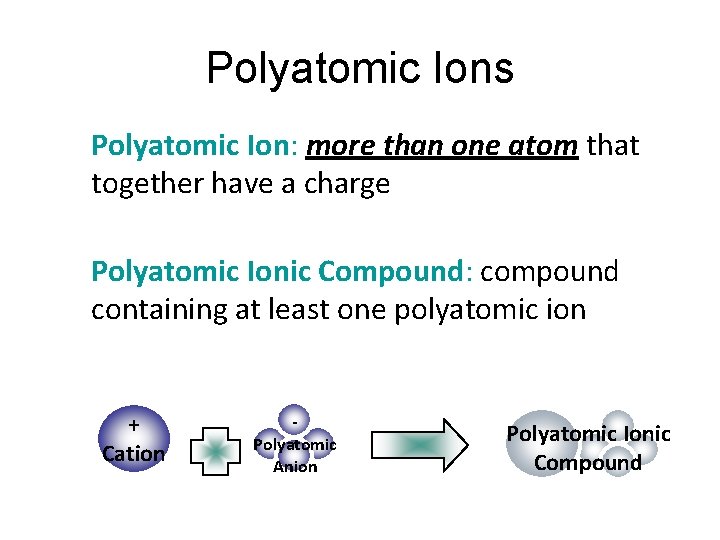
Polyatomic Ions Polyatomic Ion: more than one atom that together have a charge Polyatomic Ionic Compound: compound containing at least one polyatomic ion + Cation Polyatomic Anion Polyatomic Ionic Compound
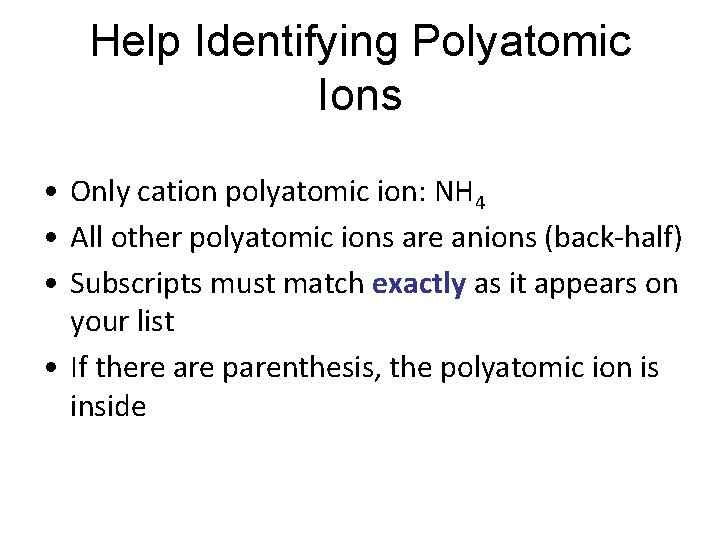
Help Identifying Polyatomic Ions • Only cation polyatomic ion: NH 4 • All other polyatomic ions are anions (back-half) • Subscripts must match exactly as it appears on your list • If there are parenthesis, the polyatomic ion is inside
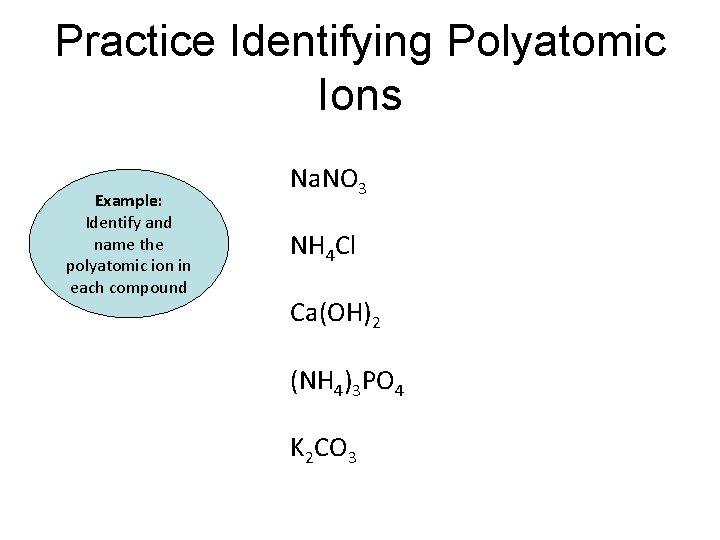
Practice Identifying Polyatomic Ions Example: Identify and name the polyatomic ion in each compound Na. NO 3 NH 4 Cl Ca(OH)2 (NH 4)3 PO 4 K 2 CO 3
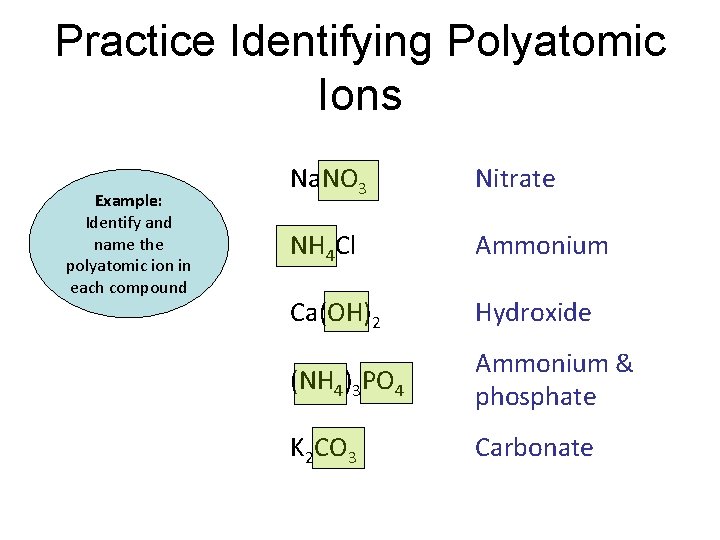
Practice Identifying Polyatomic Ions Example: Identify and name the polyatomic ion in each compound Na. NO 3 Nitrate NH 4 Cl Ammonium Ca(OH)2 Hydroxide (NH 4)3 PO 4 Ammonium & phosphate K 2 CO 3 Carbonate

Polyatomic Ionic: Formula to Name • To identify: – More than 2 capital letters (not starting with H) – at least 1 metal & 1 non-metal • To name: 1. Name of the cation 2. If the anion is a polyatomic ion, write the polyatomic ion’s name just as it is 3. If the anion is a single non-metal element, use “ide”
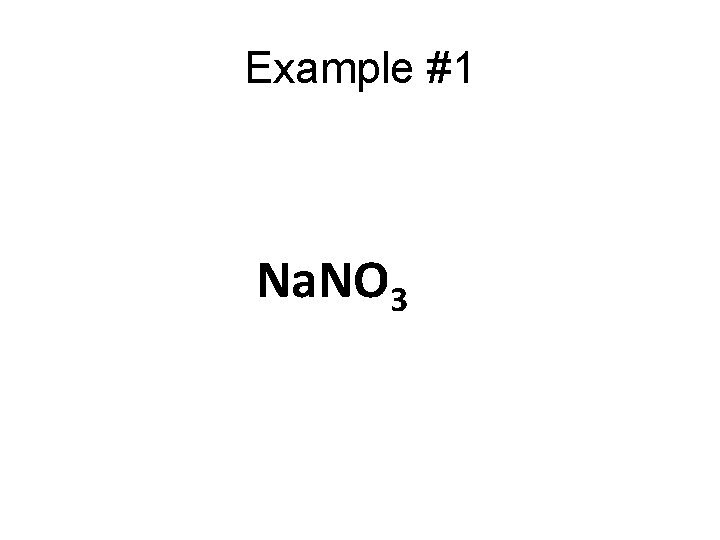
Example #1 Na. NO 3
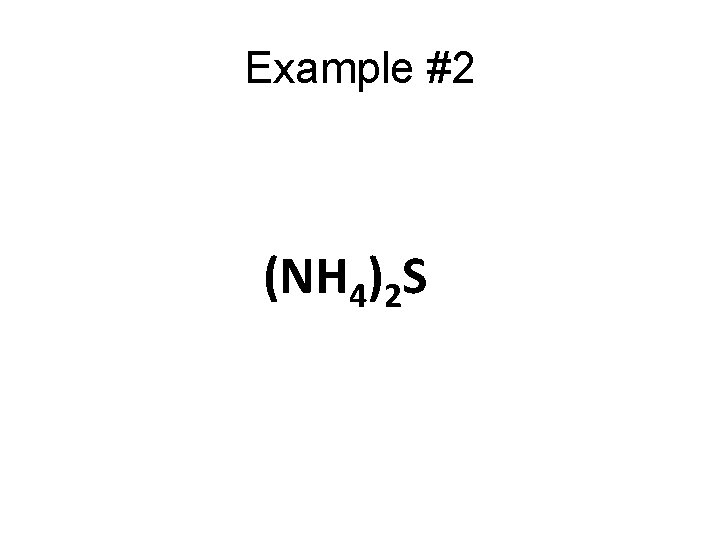
Example #2 (NH 4)2 S
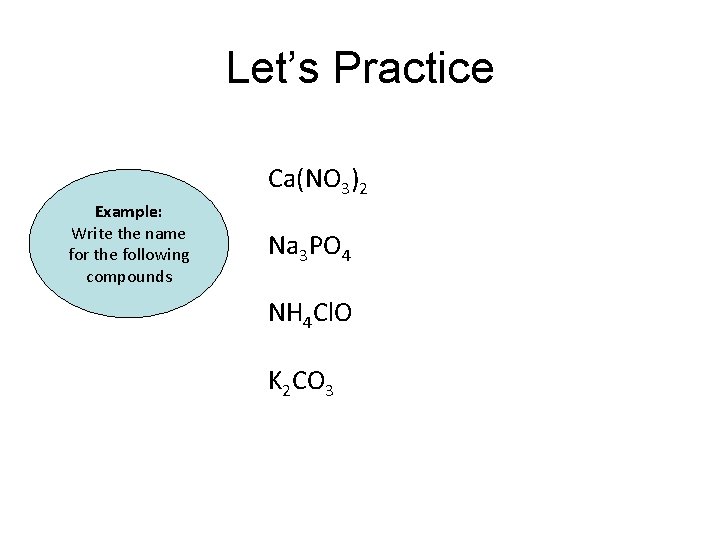
Let’s Practice Ca(NO 3)2 Example: Write the name for the following compounds Na 3 PO 4 NH 4 Cl. O K 2 CO 3

Polyatomic Ionic Compounds Name to Formula

Polyatomic Ionic: Name to Formula • To identify: – Do not end with “-ide” (except hydroxide & cyanide) – Do not use covalent prefixes • To write these formulas: 1. Write the symbol & charge of the cation & anion 2. Add additional cations or anions to have a neutral compound 3. Use subscripts to show the number of ions - When using subscripts with a polyatomic ion, you must put the polyatomic ion in parenthesis.

Example #3 Sodium carbonate

Example #4 Magnesium nitrate

Let’s Practice Example: Write the following chemical formulas Sodium nitrate Calcium chlorate Potassium sulfite Calcium hydroxide
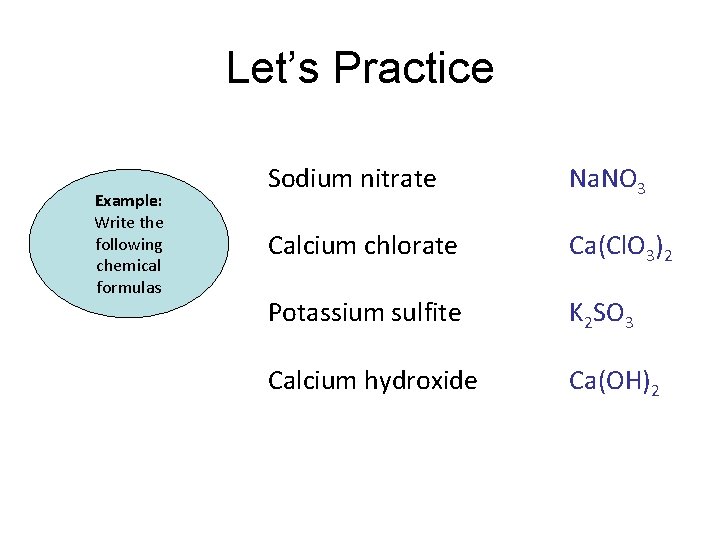
Let’s Practice Example: Write the following chemical formulas Sodium nitrate Na. NO 3 Calcium chlorate Ca(Cl. O 3)2 Potassium sulfite K 2 SO 3 Calcium hydroxide Ca(OH)2
- Slides: 42