Binary Ionic Compounds Compounds composed of two or

Binary Ionic Compounds �Compounds composed of two or more elements are binary ionic or binary molecular compounds �In binary ionic compounds the total positive and negative charges must be equal �Magnesium and bromine combine to form �Magnesium bromide: Mg 2+ Br - Mg. Br 2
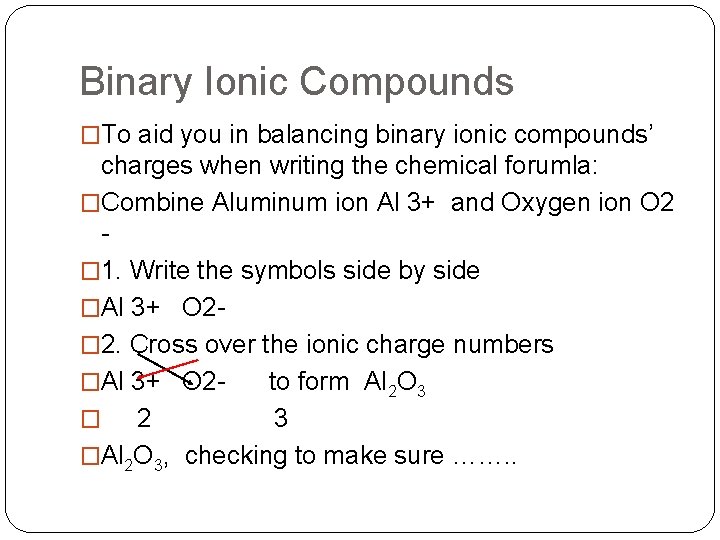
Binary Ionic Compounds �To aid you in balancing binary ionic compounds’ charges when writing the chemical forumla: �Combine Aluminum ion Al 3+ and Oxygen ion O 2 � 1. Write the symbols side by side �Al 3+ O 2� 2. Cross over the ionic charge numbers �Al 3+ O 2 to form Al 2 O 3 � 2 3 �Al 2 O 3, checking to make sure ……. .
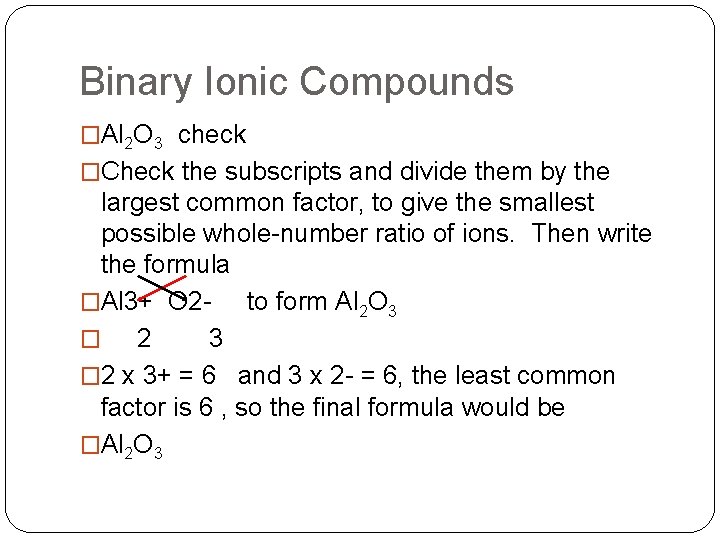
Binary Ionic Compounds �Al 2 O 3 check �Check the subscripts and divide them by the largest common factor, to give the smallest possible whole-number ratio of ions. Then write the formula �Al 3+ O 2 to form Al 2 O 3 � 2 x 3+ = 6 and 3 x 2 - = 6, the least common factor is 6 , so the final formula would be �Al 2 O 3

Binary Ionic Compounds �When naming binary ionic compounds the name of the CATION is always first, followed by the anion. �So, Al 2 O 3 �Would be Aluminum Oxide, because Oxygen is the anion.

Practice �Write the chemical formula for the following: � iron(III)oxide �Fe 2 O 3 �Calcium Sulfide �Ca. S �Barium Sulfide �Ba. S �Calcium Nitride �Ca 3 N 2

Practice continued �Lithium Oxide �Li 2 O �Copper(II) Iodide �Cu. I 2 �Write the chemical name �Na. I �Sodium Iodide K 2 S Potassium Sulfide

More practice �Write the chemical name �Ca. I 2 �Calcium Iodide �Mg. S �Magnesium Sulfide �Li. Cl �Lithium chloride
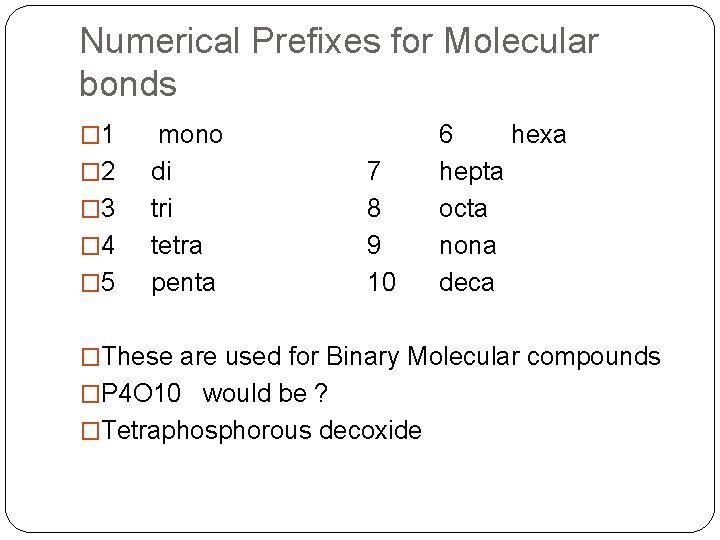
Numerical Prefixes for Molecular bonds � 1 � 2 � 3 � 4 � 5 mono di tri tetra penta 7 8 9 10 6 hexa hepta octa nona deca �These are used for Binary Molecular compounds �P 4 O 10 would be ? �Tetraphosphorous decoxide
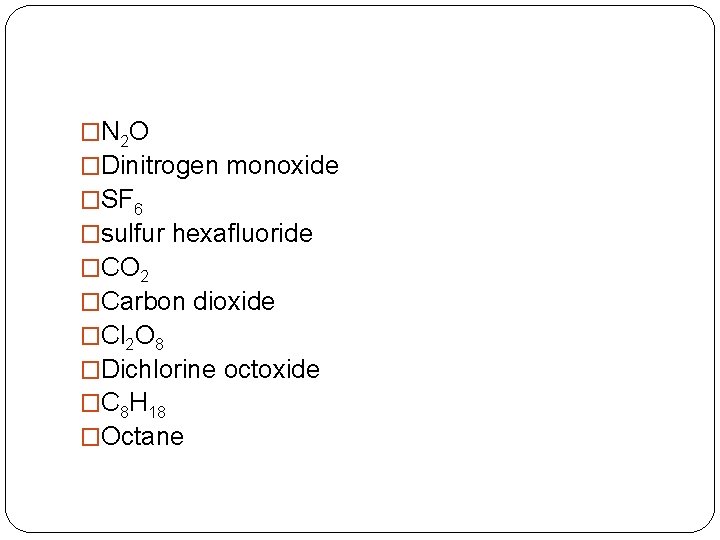
�N 2 O �Dinitrogen monoxide �SF 6 �sulfur hexafluoride �CO 2 �Carbon dioxide �Cl 2 O 8 �Dichlorine octoxide �C 8 H 18 �Octane

Homework/Classwork �In the book, page 223 chapter 7, practice problems �And �Page 225 practice problems
- Slides: 10