Binary eutectic system example silvercopper The two constituents
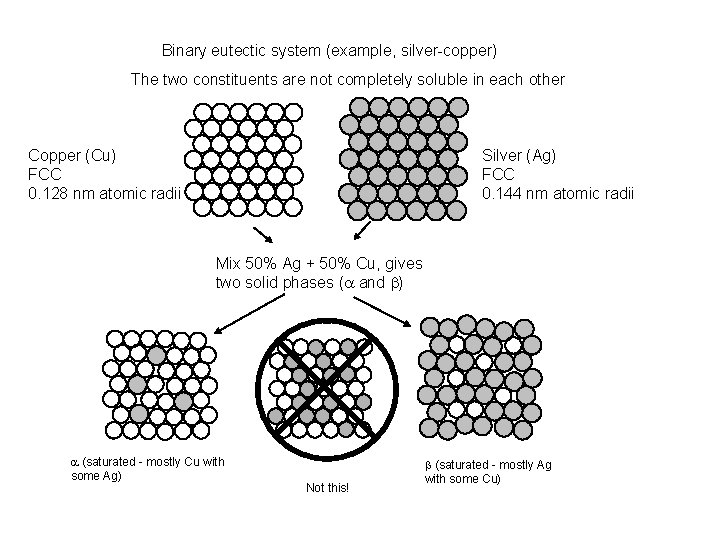
Binary eutectic system (example, silver-copper) The two constituents are not completely soluble in each other Copper (Cu) FCC 0. 128 nm atomic radii Silver (Ag) FCC 0. 144 nm atomic radii Mix 50% Ag + 50% Cu, gives two solid phases (a and b) a (saturated - mostly Cu with some Ag) Not this! b (saturated - mostly Ag with some Cu)
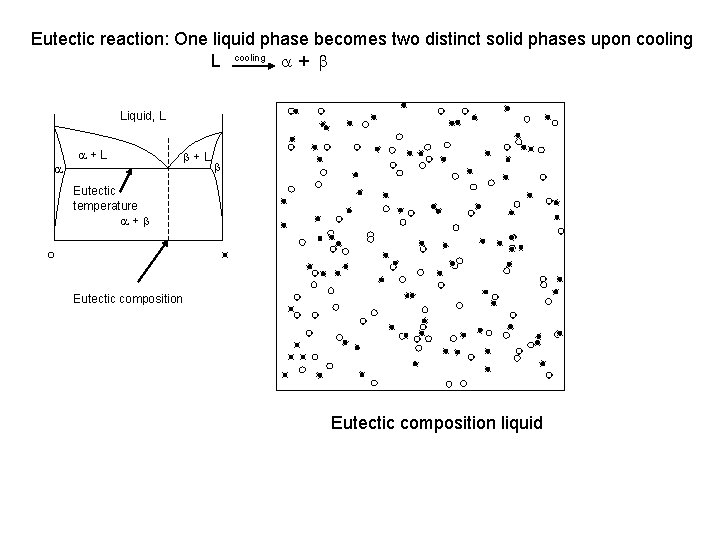
Eutectic reaction: One liquid phase becomes two distinct solid phases upon cooling L cooling a + b Liquid, L a a+L b Eutectic temperature a+b Eutectic composition liquid
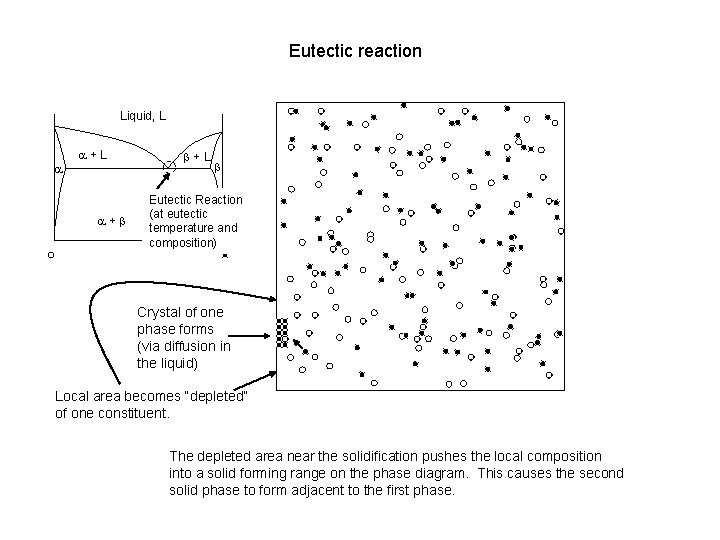
Eutectic reaction Liquid, L a a+L a+b b+L b Eutectic Reaction (at eutectic temperature and composition) Crystal of one phase forms (via diffusion in the liquid) Local area becomes “depleted” of one constituent. The depleted area near the solidification pushes the local composition into a solid forming range on the phase diagram. This causes the second solid phase to form adjacent to the first phase.
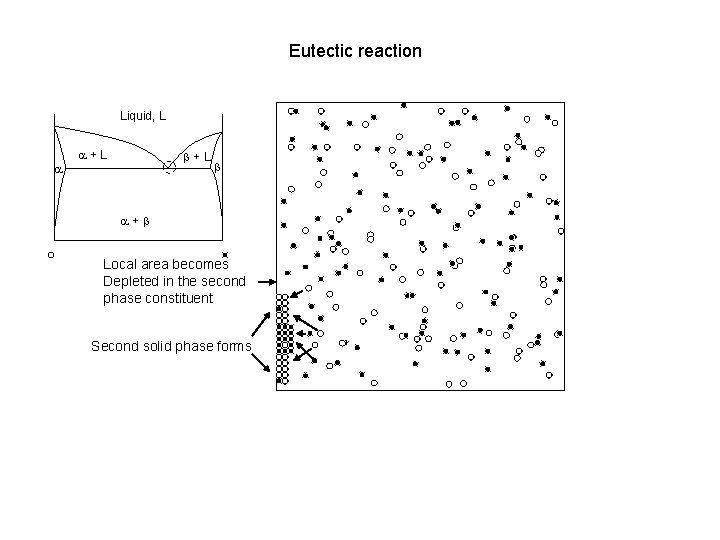
Eutectic reaction Liquid, L a a+L b a+b Local area becomes Depleted in the second phase constituent Second solid phase forms
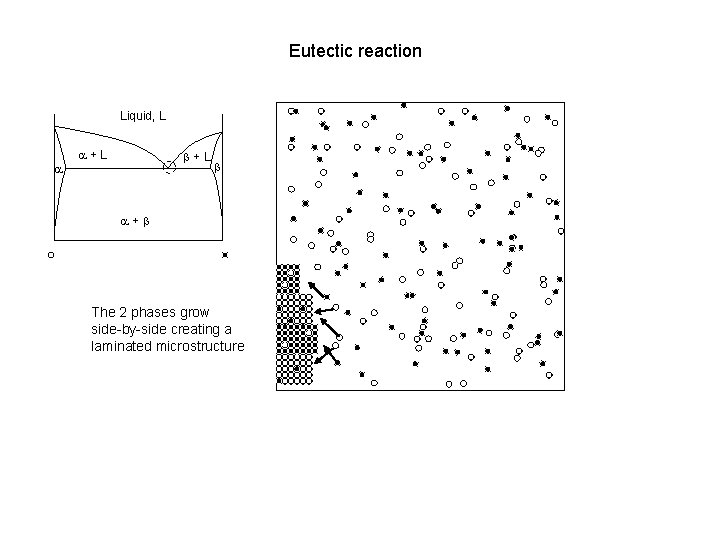
Eutectic reaction Liquid, L a a+L b a+b The 2 phases grow side-by-side creating a laminated microstructure
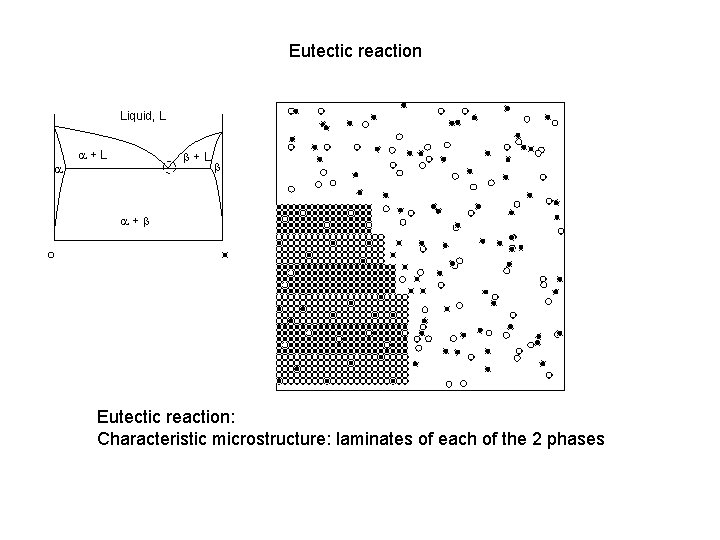
Eutectic reaction Liquid, L a a+L b a+b Eutectic reaction: Characteristic microstructure: laminates of each of the 2 phases
- Slides: 6