BINARY COMPOUNDS POLYATOMIC COMPOUNDS BINARY COMPOUNDS two elements

BINARY COMPOUNDS & POLYATOMIC COMPOUNDS
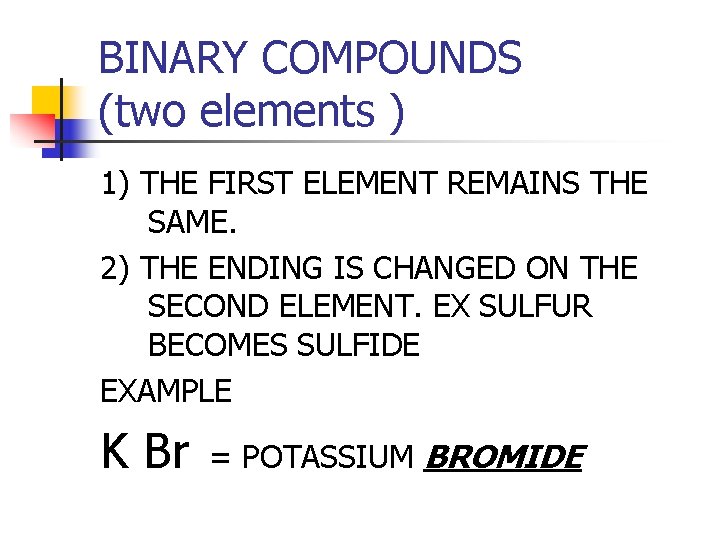
BINARY COMPOUNDS (two elements ) 1) THE FIRST ELEMENT REMAINS THE SAME. 2) THE ENDING IS CHANGED ON THE SECOND ELEMENT. EX SULFUR BECOMES SULFIDE EXAMPLE K Br = POTASSIUM BROMIDE

All of the negative elements change their endings to -ide CHLORINE = CHLORIDE BROMINE = BROMIDE FLUORINE = FLUORIDE IODINE = IODIDE OXYGEN = OXIDE SULFUR = SULFIDE NITROGEN = NITRIDE PHOSPHORUS = PHOSPHIDE CARBON= CARBIDE HYDROGEN = HYDRIDE
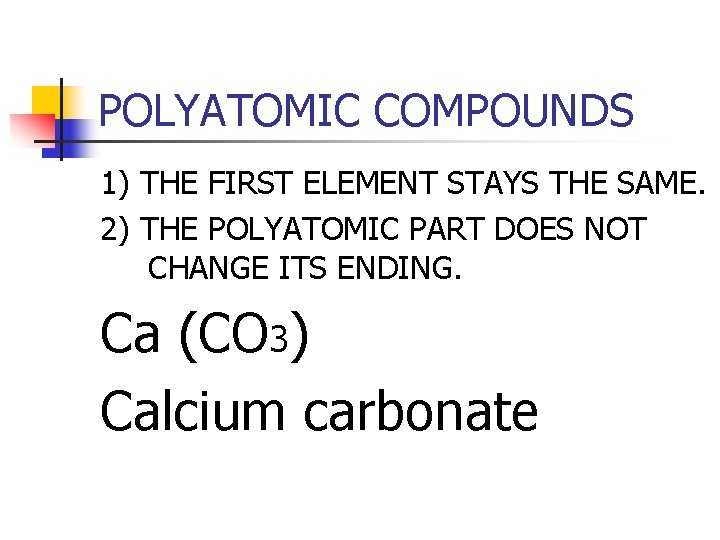
POLYATOMIC COMPOUNDS 1) THE FIRST ELEMENT STAYS THE SAME. 2) THE POLYATOMIC PART DOES NOT CHANGE ITS ENDING. Ca (CO 3) Calcium carbonate

1) Al 2 S 3 = 2) Ca Br 2 = 3) K Cl = 4) Li 2 O = 5) Ca Cl 2 =

1) Al 2 S 3 = aluminum sulfide 2) Ca Br 2 = calcium bromide 3) K Cl = potassium chloride 4) Li 2 O = lithium oxide 5) Ca Cl 2 = calcium chloride
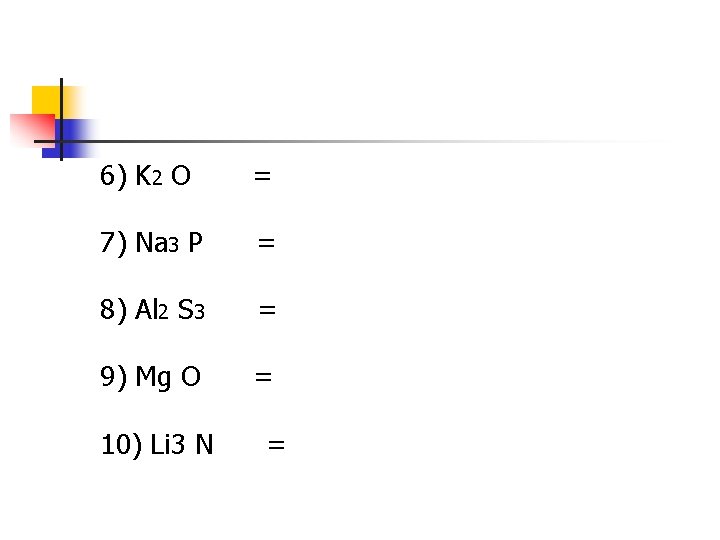
6) K 2 O = 7) Na 3 P = 8) Al 2 S 3 = 9) Mg O = 10) Li 3 N =
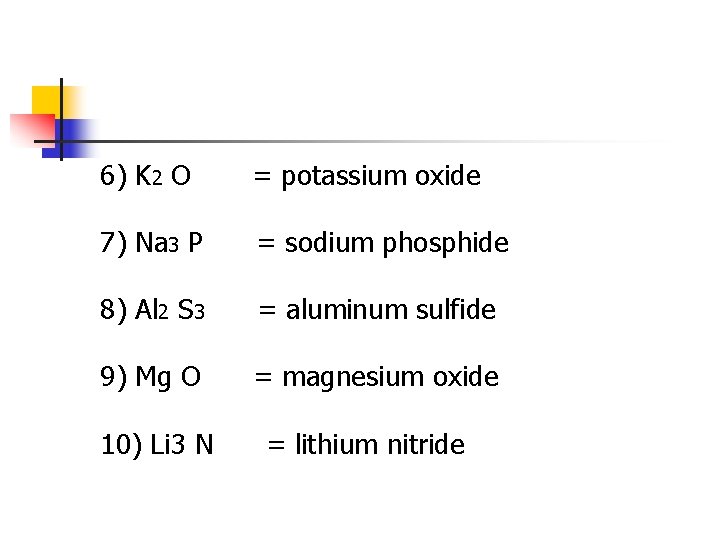
6) K 2 O = potassium oxide 7) Na 3 P = sodium phosphide 8) Al 2 S 3 = aluminum sulfide 9) Mg O = magnesium oxide 10) Li 3 N = lithium nitride
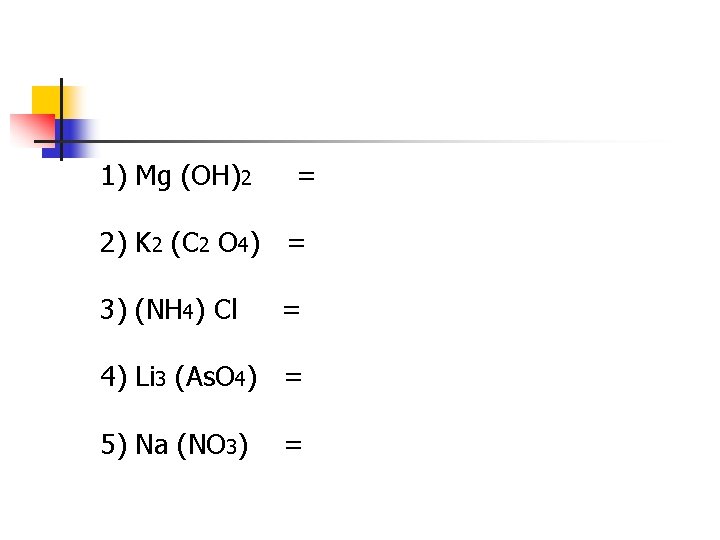
1) Mg (OH)2 = 2) K 2 (C 2 O 4) = 3) (NH 4) Cl = 4) Li 3 (As. O 4) = 5) Na (NO 3) =
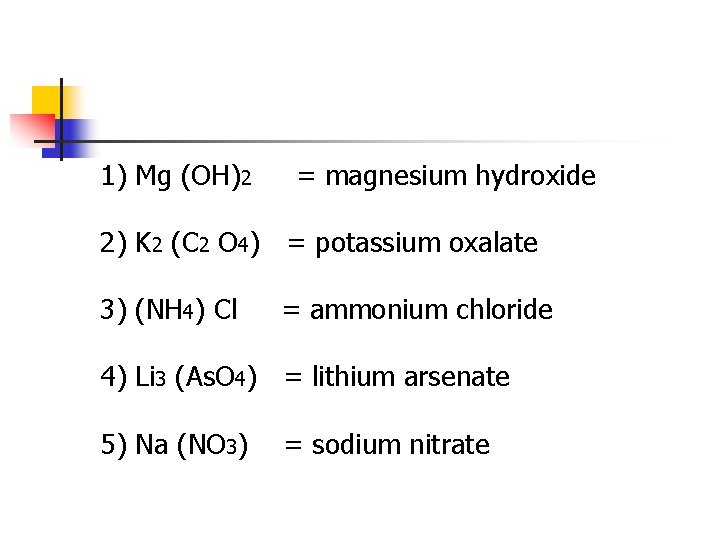
1) Mg (OH)2 = magnesium hydroxide 2) K 2 (C 2 O 4) = potassium oxalate 3) (NH 4) Cl = ammonium chloride 4) Li 3 (As. O 4) = lithium arsenate 5) Na (NO 3) = sodium nitrate
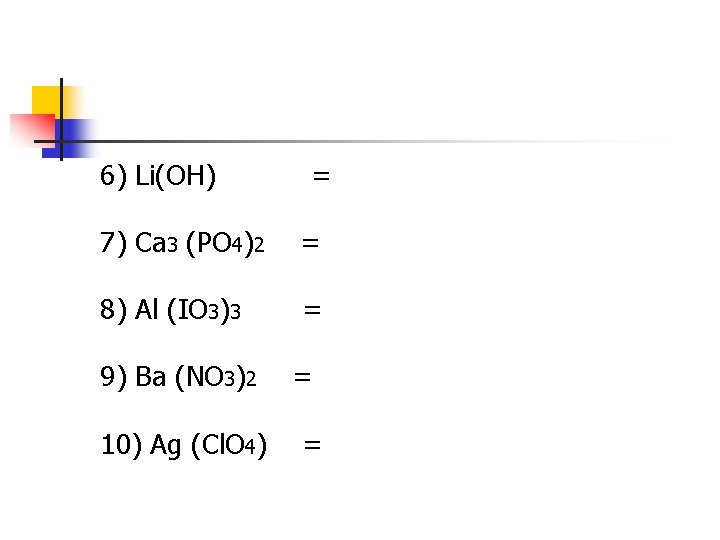
6) Li(OH) = 7) Ca 3 (PO 4)2 = 8) Al (IO 3)3 = 9) Ba (NO 3)2 10) Ag (Cl. O 4) = =
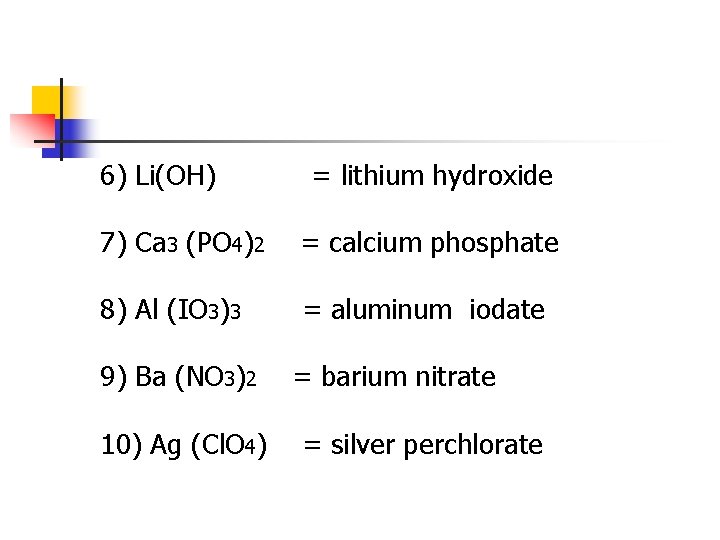
6) Li(OH) = lithium hydroxide 7) Ca 3 (PO 4)2 = calcium phosphate 8) Al (IO 3)3 = aluminum iodate 9) Ba (NO 3)2 10) Ag (Cl. O 4) = barium nitrate = silver perchlorate

HOMEWORK WS 1 – 20 BINARY CONVERT THE FOLLOWING INTO WORDS WS 1 – 20 POLY CONVERT THE FOLLOWING INTO WORDS

PRACTICE QUIZ FORMULA TO NAME 1) MAGNESIUM SULFIDE 2) ALUMINUM NITRIDE 3) CHROMIUM OXIDE 4) BISMUTH CHLORIDE 5) COBALT FLUORIDE

PRACTICE QUIZ FORMULA TO NAME 6) LITHIUM HYDROXIDE 7) CALCIUM PHOSPHATE 8) ALUMINUM IODATE 9) BARIUM HYDROXIDE 10) SILVER NITRATE
- Slides: 15