Binary Compounds Containing a Metal of Variable Oxidation
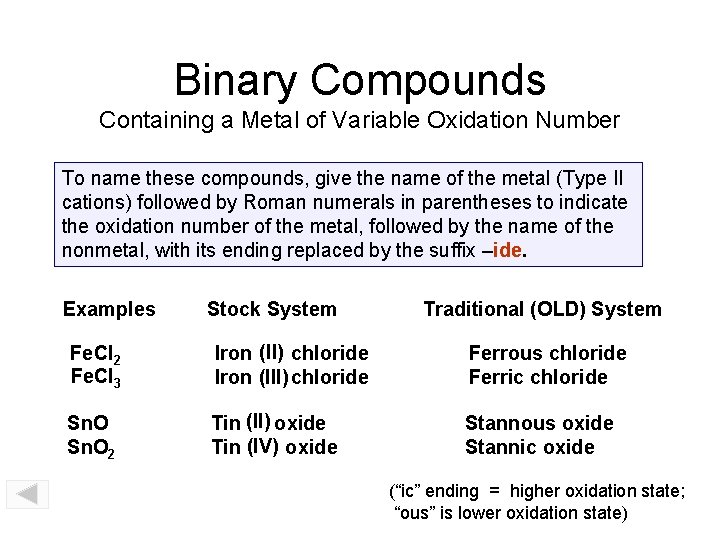
Binary Compounds Containing a Metal of Variable Oxidation Number To name these compounds, give the name of the metal (Type II cations) followed by Roman numerals in parentheses to indicate the oxidation number of the metal, followed by the name of the nonmetal, with its ending replaced by the suffix –ide. Examples Stock System Traditional (OLD) System Fe. Cl 2 Fe. Cl 3 Iron (II) chloride Iron (III) chloride Ferrous chloride Ferric chloride Sn. O 2 Tin (II) oxide Tin (IV) oxide Stannous oxide Stannic oxide (“ic” ending = higher oxidation state; “ous” is lower oxidation state)
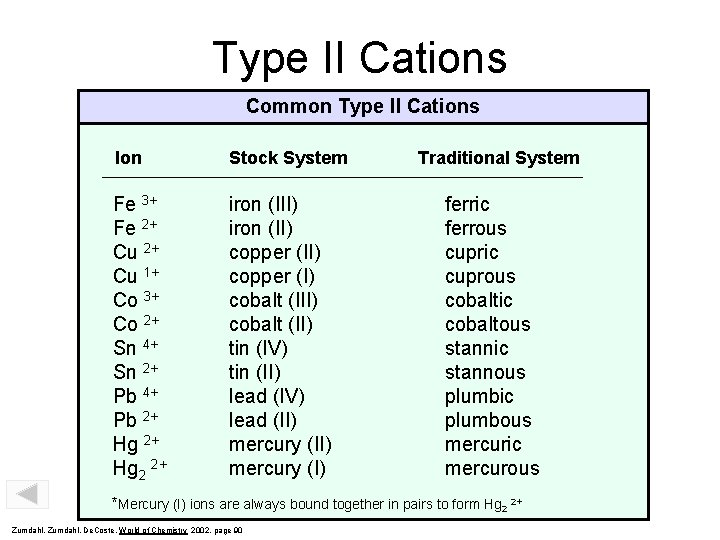
Type II Cations Common Type II Cations Ion Stock System Fe 3+ Fe 2+ Cu 1+ Co 3+ Co 2+ Sn 4+ Sn 2+ Pb 4+ Pb 2+ Hg 2 2+ iron (III) iron (II) copper (I) cobalt (II) tin (IV) tin (II) lead (IV) lead (II) mercury (I) Traditional System ferric ferrous cupric cuprous cobaltic cobaltous stannic stannous plumbic plumbous mercuric mercurous *Mercury (I) ions are always bound together in pairs to form Hg 2 2+ Zumdahl, De. Coste, World of Chemistry 2002, page 90
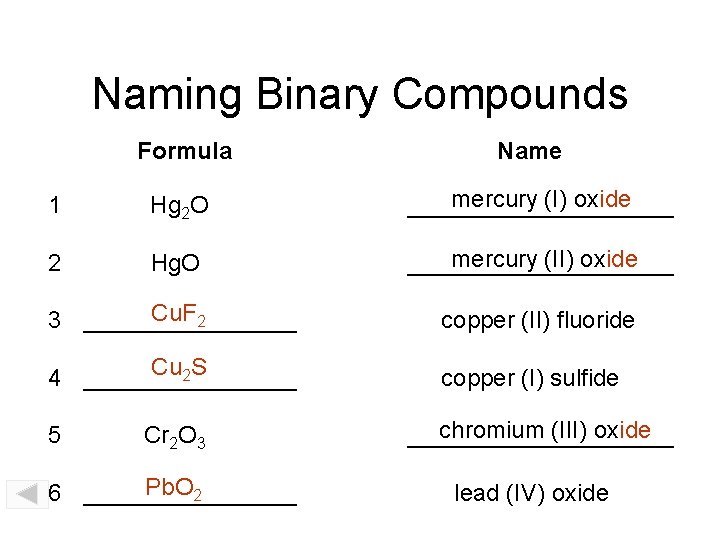
Naming Binary Compounds Formula Name 1 Hg 2 O mercury (I) oxide __________ 2 Hg. O mercury (II) oxide __________ Cu. F 2 3 ________ copper (II) fluoride Cu 2 S 4 ________ copper (I) sulfide 5 Cr 2 O 3 Pb. O 2 6 ________ chromium (III) oxide __________ lead (IV) oxide
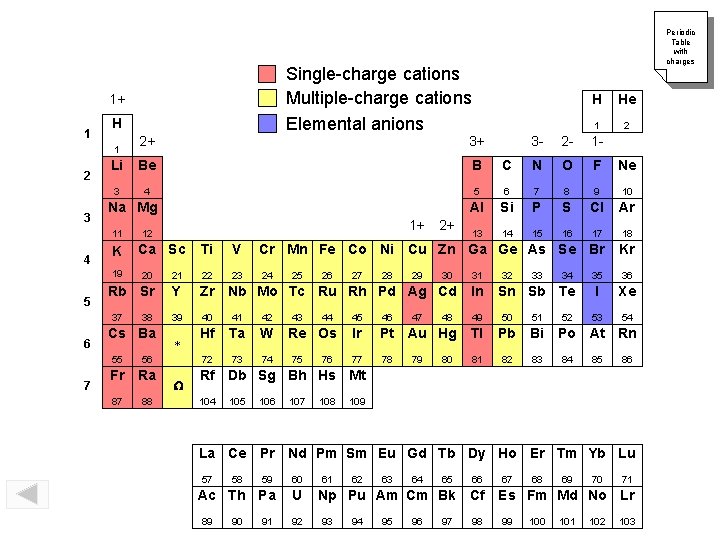
Single-charge cations Multiple-charge cations Elemental anions 1+ 1 H 2+ 3+ Li Be B 3 4 1 2 3 Na Mg 11 4 K 19 5 7 1+ 12 Ca Sc 2+ N O F Ne 5 6 7 8 9 10 Al Si P S Cl Ar 13 14 15 16 17 18 Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 23 24 35 36 I Xe 53 54 Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 39 40 41 42 49 50 51 Hf Ta W 72 73 74 55 56 Fr Ra 87 88 W 2 C Rb Sr * 1 1 - 22 Cs Ba He 2 - 21 38 H 3 - 20 37 6 Periodic Table with charges 25 43 26 44 Re Os 75 76 27 28 29 47 30 32 33 46 Ir Pt Au Hg Tl Pb Bi 77 78 81 82 83 80 34 Sn Sb Te 45 79 48 31 52 Po At Rn 84 85 86 Rf Db Sg Bh Hs Mt 104 105 106 107 108 109 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 57 59 60 Ac Th Pa U 89 58 90 91 92 61 62 63 64 65 66 Np Pu Am Cm Bk Cf 93 94 95 96 97 98 67 68 69 70 71 Es Fm Md No Lr 99 100 101 102 103
- Slides: 4