Big 7 Chapter 2 2 Matter and Energy
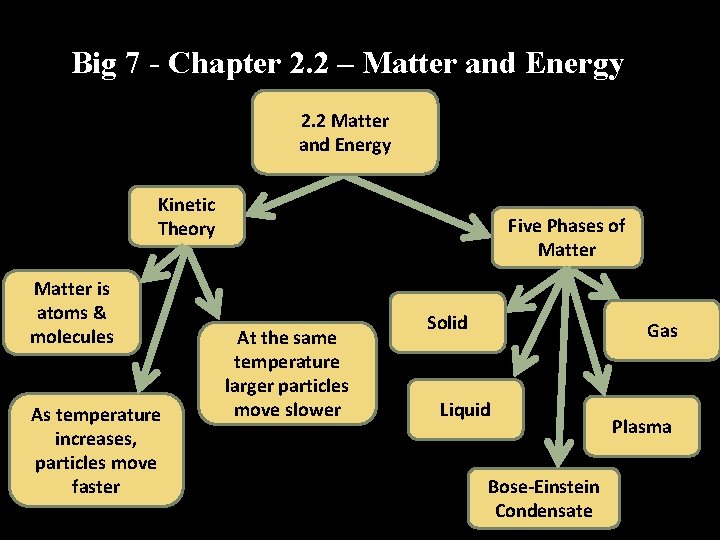
Big 7 - Chapter 2. 2 – Matter and Energy 2. 2 Matter and Energy Kinetic Theory Matter is atoms & molecules As temperature increases, particles move faster Five Phases of Matter At the same temperature larger particles move slower Solid Gas Liquid Bose-Einstein Condensate Plasma

Big 7 - Chapter 2. 2 – Matter and Energy 1. Kinetic Theory Part I 2. Kinetic Theory Part II 3. Kinetic Theory Part III 4. Properties of Solids, liquids, gases, plasmas, and Bose-Einstein condensates 5. Law: Conservation of Mass

1. Kinetic Theory (Part 1 of 3) Matter is made of atoms and molecules
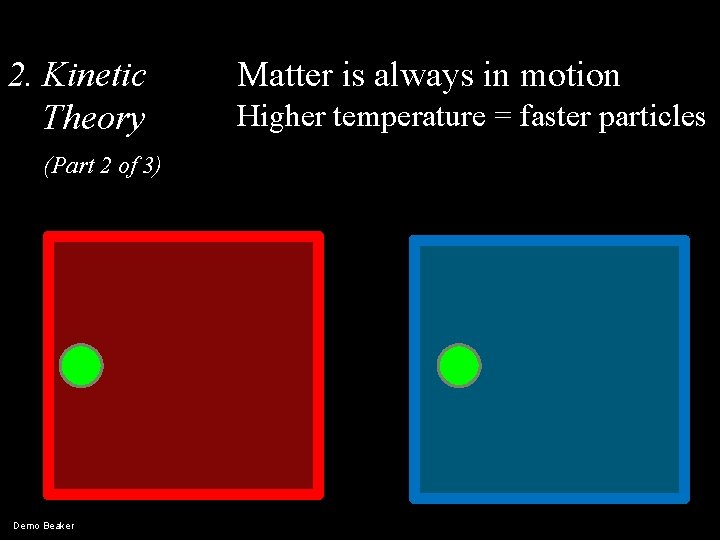
2. Kinetic Theory (Part 2 of 3) Demo Beaker Matter is always in motion Higher temperature = faster particles

Q 1. True or False – Matter can be made of substances other than atoms and molecules. TRUE FALSE
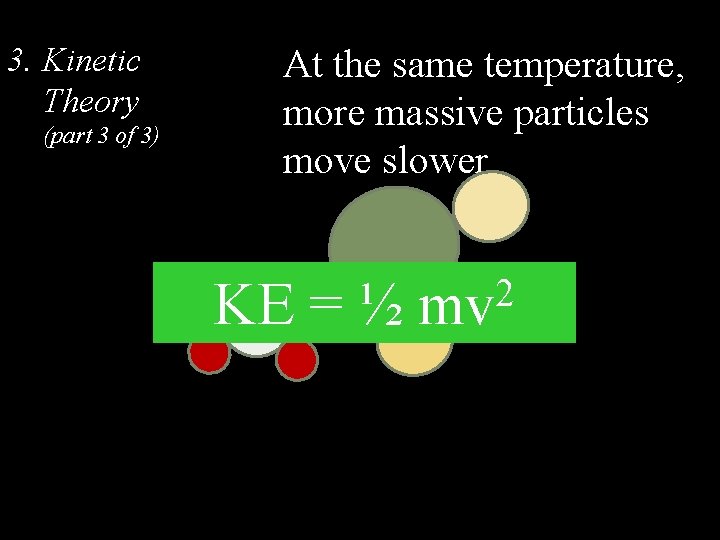
3. Kinetic Theory (part 3 of 3) At the same temperature, more massive particles move slower KE = ½ 2 mv

Q 2. Three beakers with 50 m. L of water are on the desk. The “cool” one is 10 o. C, the “warm” one is 40 o. C, and the “hot” one is 90 o. C. In which beaker are the water molecules moving the slowest? The “cool” beaker of water The “warm” beaker of water The “hot” beaker of water

Q 3. A water molecule has less mass than a sugar molecule. If Sheldon dissolves a tablespoon of sugar into a cup of water, which statement is true? The water molecules are moving faster than the sugar molecules The water molecules are moving slower than the sugar molecules The water and sugar molecules are moving at the same speed I drank the sugar water.
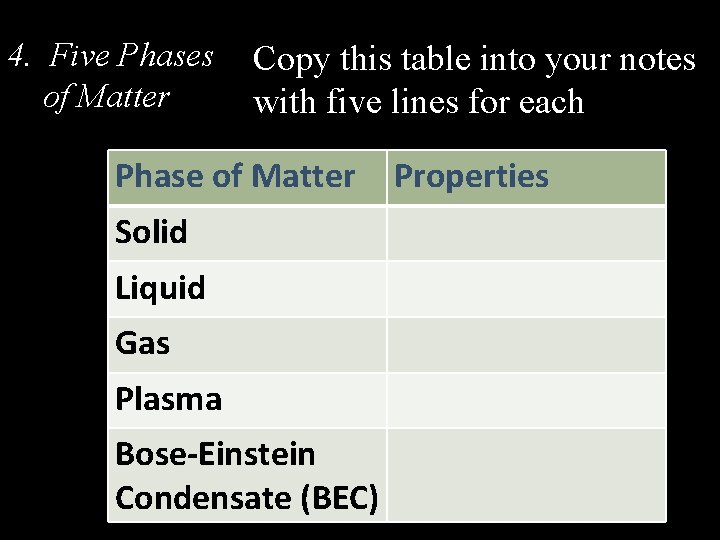
4. Five Phases of Matter Copy this table into your notes with five lines for each Phase of Matter Solid Liquid Gas Plasma Bose-Einstein Condensate (BEC) Properties
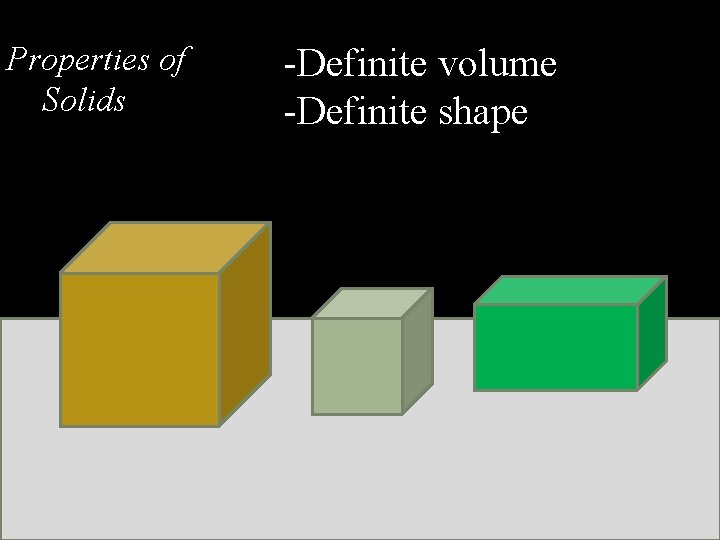
Properties of Solids -Definite volume -Definite shape
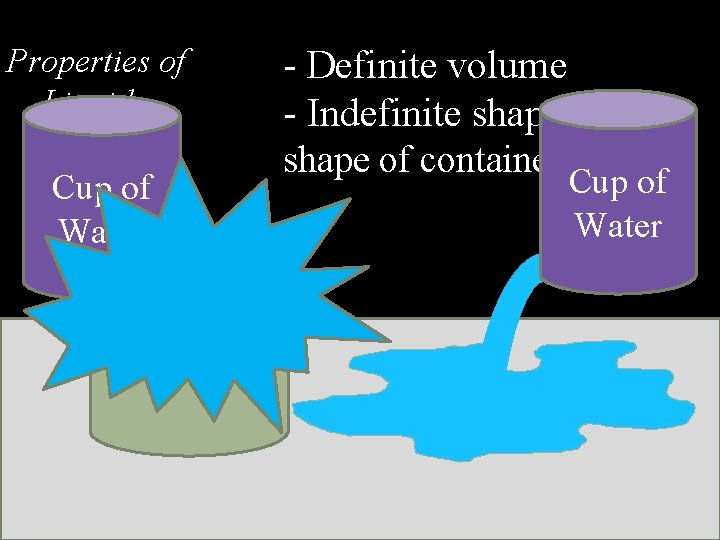
Properties of Liquids Cup of Water - Definite volume - Indefinite shape (takes shape of container) Cup of Water
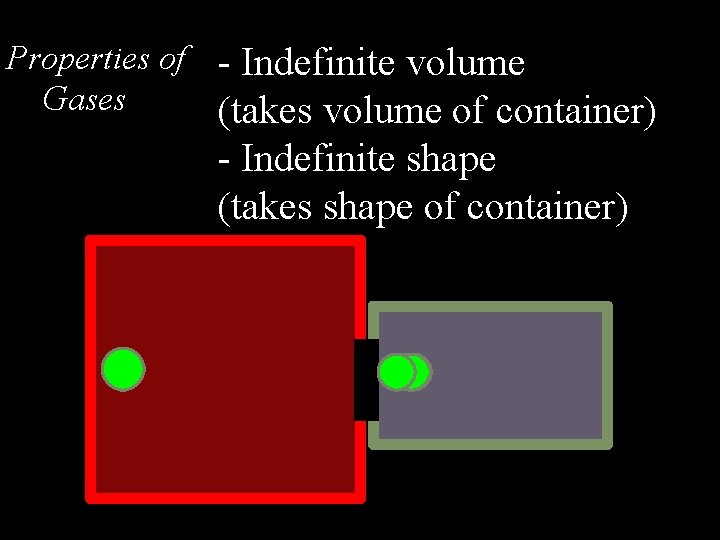
Properties of - Indefinite volume Gases (takes volume of container) - Indefinite shape (takes shape of container)

Q 4. Mrs. Green has substance that has a definite volume, but not a definite shape. What does she have? She has a solid She has a liquid She has a gas

Properties of Plasma -No definite volume or shape -Hot ionized material like gas -Electrons can move around -Conducts electricity -High energy / temperature

Q 5. Which statement is FALSE when talking about plasmas and gases They both have an indefinite volume and indefinite shape Plasmas may be created by heating or ionizing a gas, creating an electrically conductive material Plasmas are super-cold and we know very little about them
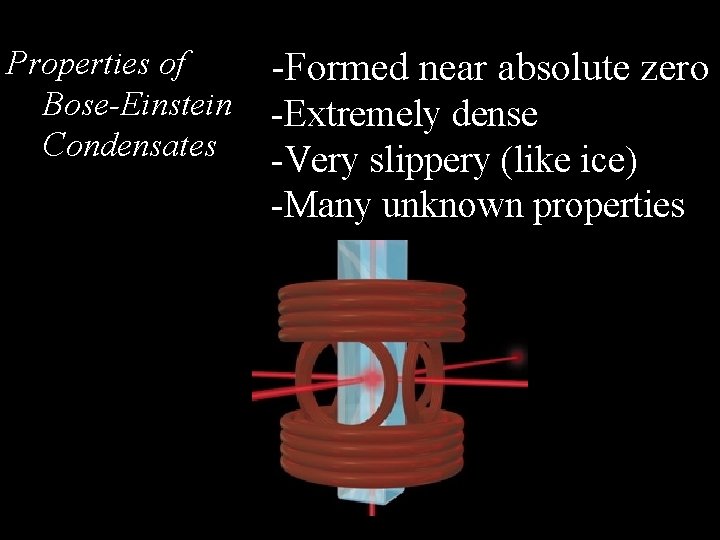
Properties of Bose-Einstein Condensates -Formed near absolute zero -Extremely dense -Very slippery (like ice) -Many unknown properties

Q 6. The only three phases of matter are solid, liquid and gas. TRUE FALSE

Q 7. Mr. Hernandez is evaluating a rock. He has classified the rock as in the solid phase. Therefore, the rock has a ____ volume and a _______ shape Indefinite, indefinite Definite, indefinite Indefinite, definite Definite, definite None of the above
Q 8. Which of the five phased do we know the least about? Solids Liquids Gases Plasmas Bose-Einstein Condensates

5. Law of Conservation of Mass cannot be created nor destroyed

Q 9. Ice and water are both made of H 2 O molecules. Which of the following statement is TRUE regarding ice and water. Molecules in the ice are moving faster than in the water Molecules in the water are moving faster than in the ice Since they are the same molecules, they are moving at the same speed

Q 10. According to the conservation of mass, mass cannot be created nor destroyed. Which of the following statements BEST describes the evaporation of a puddle in the summer? The water turns into heat which radiates and forms distorted images The water evaporates into water vapor; however, the mass of the puddle before evaporation equals the mass of the water vapor after the evaporation The water is destroyed never to be seen again
- Slides: 22