BictegravirTenofovir alafenamideEmtricitabine Biktarvy Prepared by Brian R Wood

Bictegravir-Tenofovir alafenamide-Emtricitabine (Biktarvy) Prepared by: Brian R. Wood, MD David H. Spach, MD Last Updated: December 31, 2019
![Bictegravir-Tenofovir Alafenamide-Emtricitabine (Biktarvy) Biktarvy [bik-TAR-vee] Bictegravir-Tenofovir alafenamide-Emtricitabine 50 mg 25 mg INSTI 200 mg Bictegravir-Tenofovir Alafenamide-Emtricitabine (Biktarvy) Biktarvy [bik-TAR-vee] Bictegravir-Tenofovir alafenamide-Emtricitabine 50 mg 25 mg INSTI 200 mg](http://slidetodoc.com/presentation_image/578f1954931e2c135ca8ca69df498277/image-2.jpg)
Bictegravir-Tenofovir Alafenamide-Emtricitabine (Biktarvy) Biktarvy [bik-TAR-vee] Bictegravir-Tenofovir alafenamide-Emtricitabine 50 mg 25 mg INSTI 200 mg NRTI Dose: 1 tablet once daily with or without food NRTI
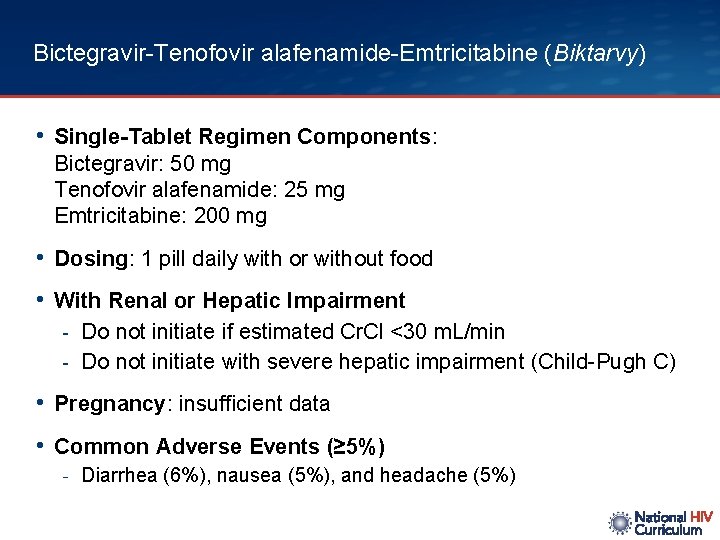
Bictegravir-Tenofovir alafenamide-Emtricitabine (Biktarvy) • Single-Tablet Regimen Components: Bictegravir: 50 mg Tenofovir alafenamide: 25 mg Emtricitabine: 200 mg • Dosing: 1 pill daily with or without food • With Renal or Hepatic Impairment - Do not initiate if estimated Cr. Cl <30 m. L/min - Do not initiate with severe hepatic impairment (Child-Pugh C) • Pregnancy: insufficient data • Common Adverse Events (≥ 5%) - Diarrhea (6%), nausea (5%), and headache (5%)
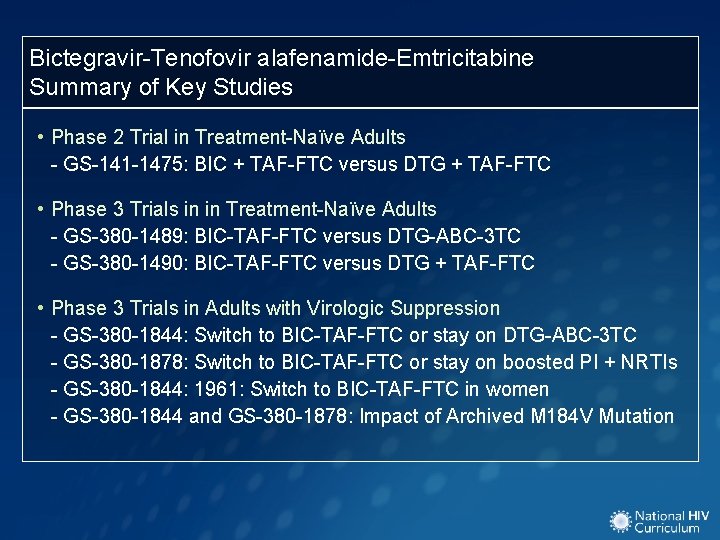
Bictegravir-Tenofovir alafenamide-Emtricitabine Summary of Key Studies • Phase 2 Trial in Treatment-Naïve Adults - GS-141 -1475: BIC + TAF-FTC versus DTG + TAF-FTC • Phase 3 Trials in in Treatment-Naïve Adults - GS-380 -1489: BIC-TAF-FTC versus DTG-ABC-3 TC - GS-380 -1490: BIC-TAF-FTC versus DTG + TAF-FTC • Phase 3 Trials in Adults with Virologic Suppression - GS-380 -1844: Switch to BIC-TAF-FTC or stay on DTG-ABC-3 TC - GS-380 -1878: Switch to BIC-TAF-FTC or stay on boosted PI + NRTIs - GS-380 -1844: 1961: Switch to BIC-TAF-FTC in women - GS-380 -1844 and GS-380 -1878: Impact of Archived M 184 V Mutation

INITIAL THERAPY Bictegravir-Tenofovir alafenamide-Emtricitabine

Bictegravir versus Dolutegravir, each with TAF-FTC GS-141 -1475
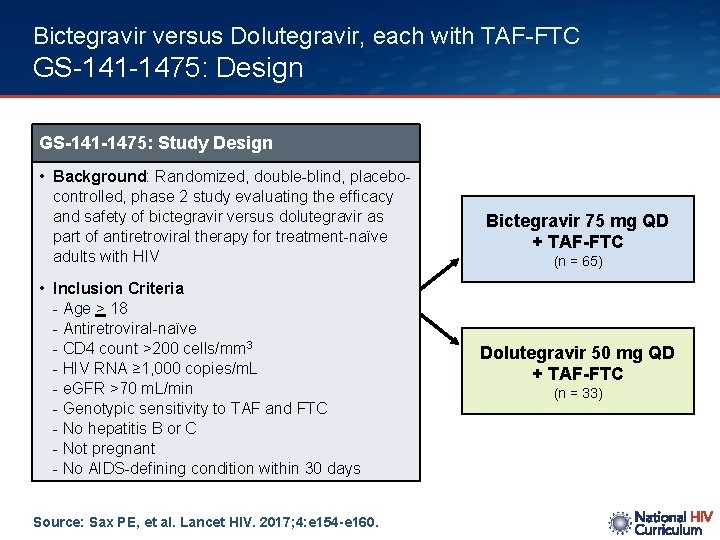
Bictegravir versus Dolutegravir, each with TAF-FTC GS-141 -1475: Design GS-141 -1475: Study Design • Background: Randomized, double-blind, placebocontrolled, phase 2 study evaluating the efficacy and safety of bictegravir versus dolutegravir as part of antiretroviral therapy for treatment-naïve adults with HIV • Inclusion Criteria - Age > 18 - Antiretroviral-naïve - CD 4 count >200 cells/mm 3 - HIV RNA ≥ 1, 000 copies/m. L - e. GFR >70 m. L/min - Genotypic sensitivity to TAF and FTC - No hepatitis B or C - Not pregnant - No AIDS-defining condition within 30 days Source: Sax PE, et al. Lancet HIV. 2017; 4: e 154 -e 160. Bictegravir 75 mg QD + TAF-FTC (n = 65) Dolutegravir 50 mg QD + TAF-FTC (n = 33)
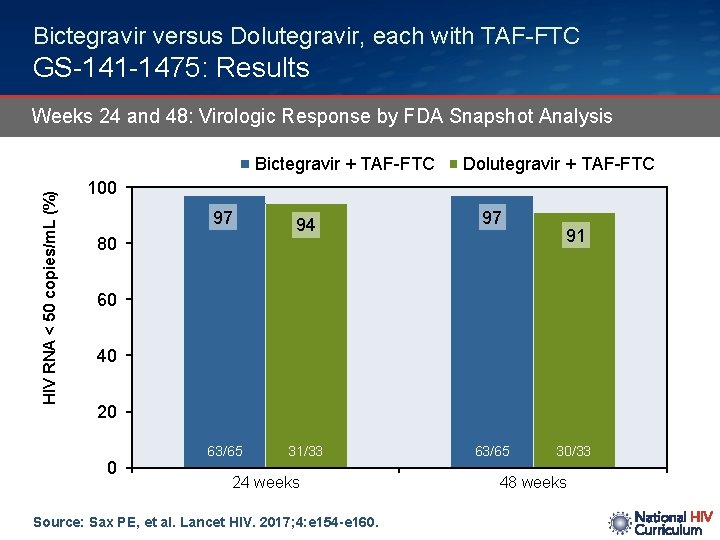
Bictegravir versus Dolutegravir, each with TAF-FTC GS-141 -1475: Results Weeks 24 and 48: Virologic Response by FDA Snapshot Analysis HIV RNA < 50 copies/m. L (%) Bictegravir + TAF-FTC Dolutegravir + TAF-FTC 100 97 80 94 97 91 60 40 20 0 63/65 31/33 24 weeks Source: Sax PE, et al. Lancet HIV. 2017; 4: e 154 -e 160. 63/65 30/33 48 weeks
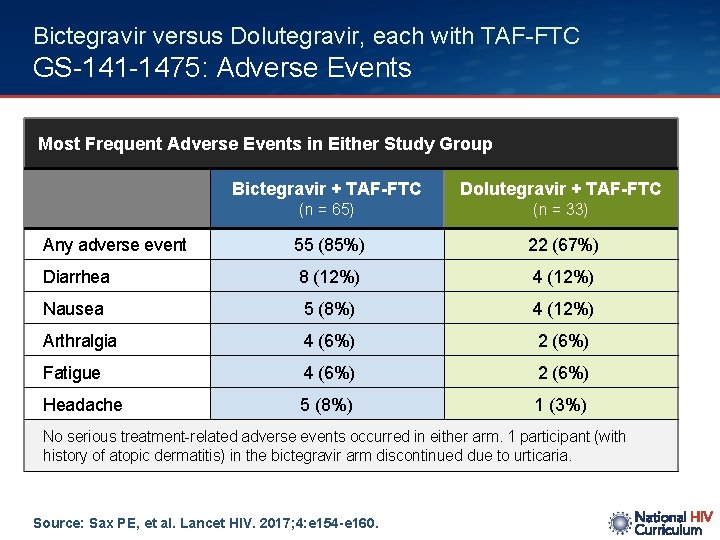
Bictegravir versus Dolutegravir, each with TAF-FTC GS-141 -1475: Adverse Events Most Frequent Adverse Events in Either Study Group Bictegravir + TAF-FTC Dolutegravir + TAF-FTC (n = 65) (n = 33) Any adverse event 55 (85%) 22 (67%) Diarrhea 8 (12%) 4 (12%) Nausea 5 (8%) 4 (12%) Arthralgia 4 (6%) 2 (6%) Fatigue 4 (6%) 2 (6%) Headache 5 (8%) 1 (3%) No serious treatment-related adverse events occurred in either arm. 1 participant (with history of atopic dermatitis) in the bictegravir arm discontinued due to urticaria. Source: Sax PE, et al. Lancet HIV. 2017; 4: e 154 -e 160.
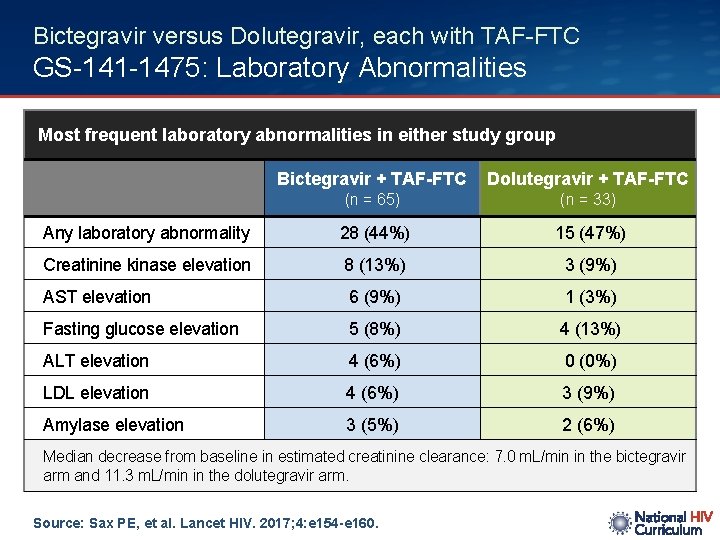
Bictegravir versus Dolutegravir, each with TAF-FTC GS-141 -1475: Laboratory Abnormalities Most frequent laboratory abnormalities in either study group Bictegravir + TAF-FTC Dolutegravir + TAF-FTC (n = 65) (n = 33) Any laboratory abnormality 28 (44%) 15 (47%) Creatinine kinase elevation 8 (13%) 3 (9%) AST elevation 6 (9%) 1 (3%) Fasting glucose elevation 5 (8%) 4 (13%) ALT elevation 4 (6%) 0 (0%) LDL elevation 4 (6%) 3 (9%) Amylase elevation 3 (5%) 2 (6%) Median decrease from baseline in estimated creatinine clearance: 7. 0 m. L/min in the bictegravir arm and 11. 3 m. L/min in the dolutegravir arm. Source: Sax PE, et al. Lancet HIV. 2017; 4: e 154 -e 160.
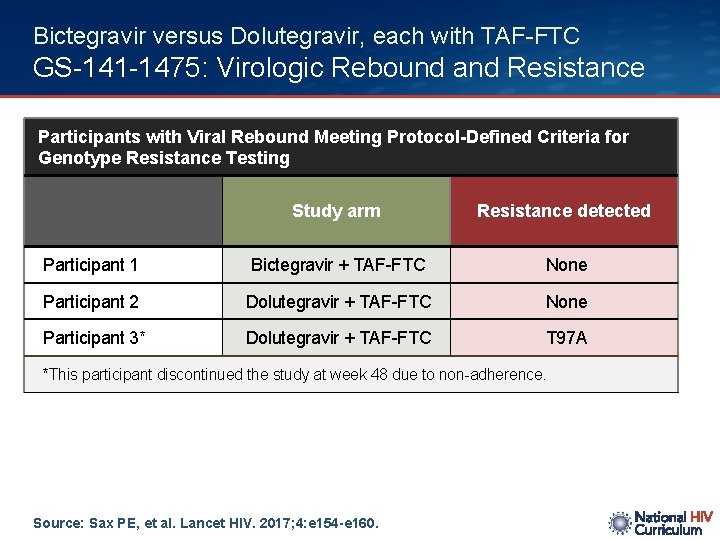
Bictegravir versus Dolutegravir, each with TAF-FTC GS-141 -1475: Virologic Rebound and Resistance Participants with Viral Rebound Meeting Protocol-Defined Criteria for Genotype Resistance Testing Study arm Resistance detected Participant 1 Bictegravir + TAF-FTC None Participant 2 Dolutegravir + TAF-FTC None Participant 3* Dolutegravir + TAF-FTC T 97 A *This participant discontinued the study at week 48 due to non-adherence. Source: Sax PE, et al. Lancet HIV. 2017; 4: e 154 -e 160.
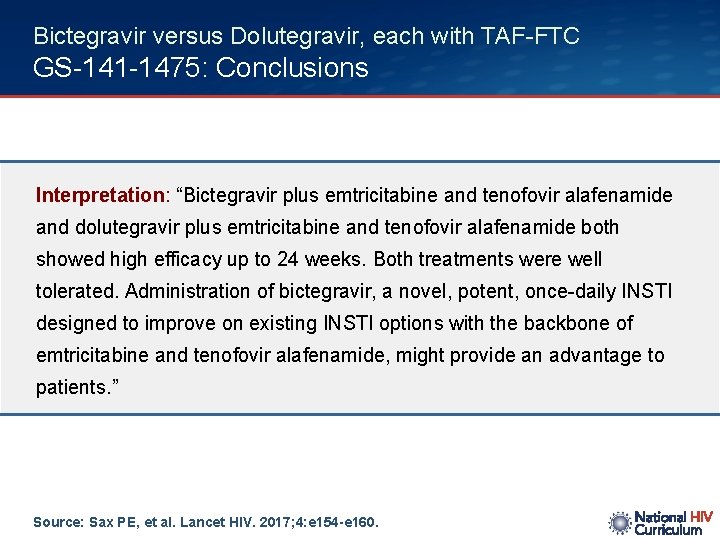
Bictegravir versus Dolutegravir, each with TAF-FTC GS-141 -1475: Conclusions Interpretation: “Bictegravir plus emtricitabine and tenofovir alafenamide and dolutegravir plus emtricitabine and tenofovir alafenamide both showed high efficacy up to 24 weeks. Both treatments were well tolerated. Administration of bictegravir, a novel, potent, once-daily INSTI designed to improve on existing INSTI options with the backbone of emtricitabine and tenofovir alafenamide, might provide an advantage to patients. ” Source: Sax PE, et al. Lancet HIV. 2017; 4: e 154 -e 160.

BIC-TAF-FTC vs. DTG-ABC-3 TC as Initial Therapy GS-380 -1489
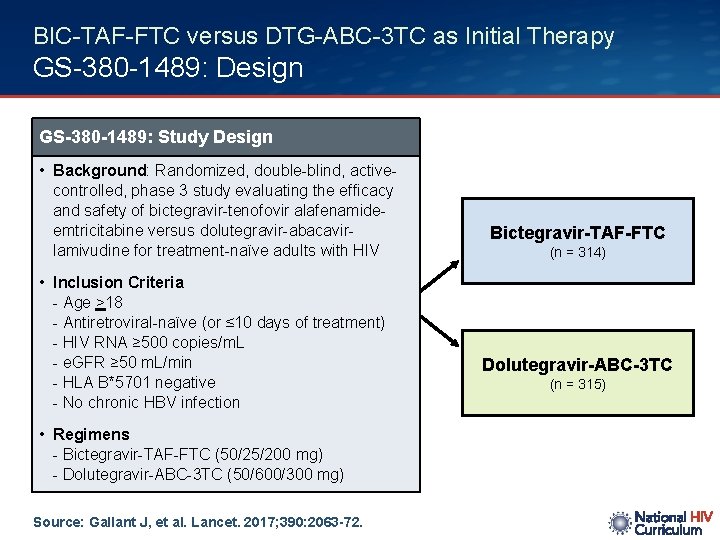
BIC-TAF-FTC versus DTG-ABC-3 TC as Initial Therapy GS-380 -1489: Design GS-380 -1489: Study Design • Background: Randomized, double-blind, activecontrolled, phase 3 study evaluating the efficacy and safety of bictegravir-tenofovir alafenamideemtricitabine versus dolutegravir-abacavirlamivudine for treatment-naïve adults with HIV • Inclusion Criteria - Age >18 - Antiretroviral-naïve (or ≤ 10 days of treatment) - HIV RNA ≥ 500 copies/m. L - e. GFR ≥ 50 m. L/min - HLA B*5701 negative - No chronic HBV infection • Regimens - Bictegravir-TAF-FTC (50/25/200 mg) - Dolutegravir-ABC-3 TC (50/600/300 mg) Source: Gallant J, et al. Lancet. 2017; 390: 2063 -72. Bictegravir-TAF-FTC (n = 314) Dolutegravir-ABC-3 TC (n = 315)
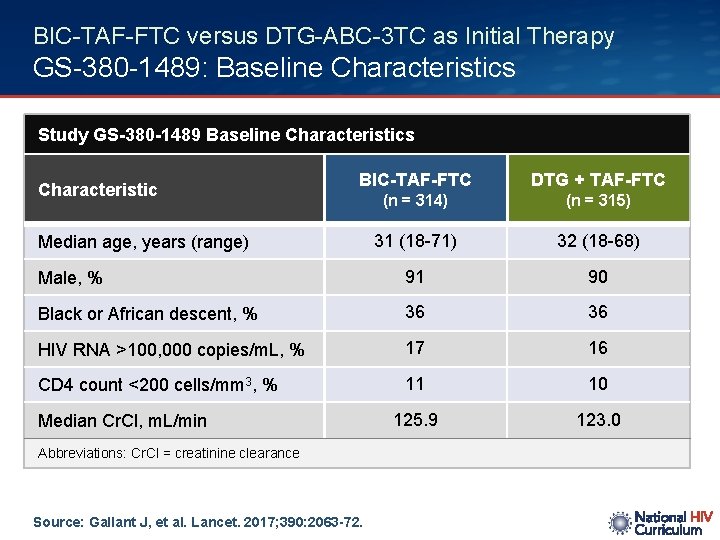
BIC-TAF-FTC versus DTG-ABC-3 TC as Initial Therapy GS-380 -1489: Baseline Characteristics Study GS-380 -1489 Baseline Characteristics BIC-TAF-FTC DTG + TAF-FTC (n = 314) (n = 315) 31 (18 -71) 32 (18 -68) Male, % 91 90 Black or African descent, % 36 36 HIV RNA >100, 000 copies/m. L, % 17 16 CD 4 count <200 cells/mm 3, % 11 10 125. 9 123. 0 Characteristic Median age, years (range) Median Cr. Cl, m. L/min Abbreviations: Cr. Cl = creatinine clearance Source: Gallant J, et al. Lancet. 2017; 390: 2063 -72.
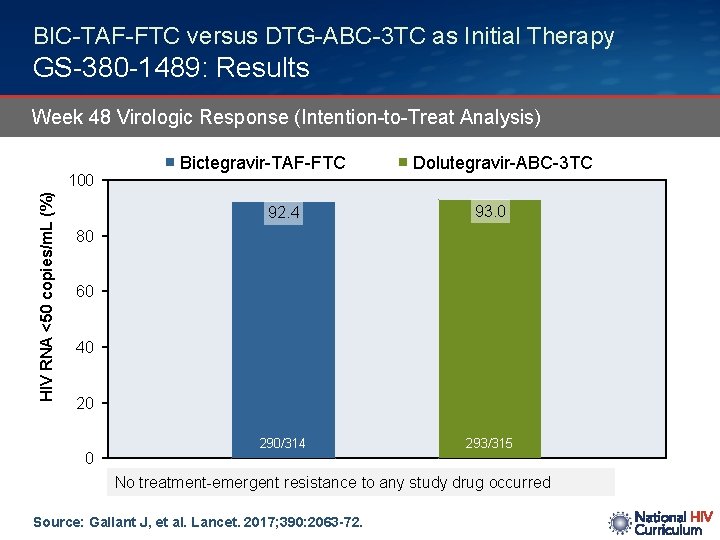
BIC-TAF-FTC versus DTG-ABC-3 TC as Initial Therapy GS-380 -1489: Results Week 48 Virologic Response (Intention-to-Treat Analysis) HIV RNA <50 copies/m. L (%) 100 Bictegravir-TAF-FTC Dolutegravir-ABC-3 TC 92. 4 93. 0 290/314 293/315 80 60 40 20 0 No treatment-emergent resistance to any study drug occurred Source: Gallant J, et al. Lancet. 2017; 390: 2063 -72.
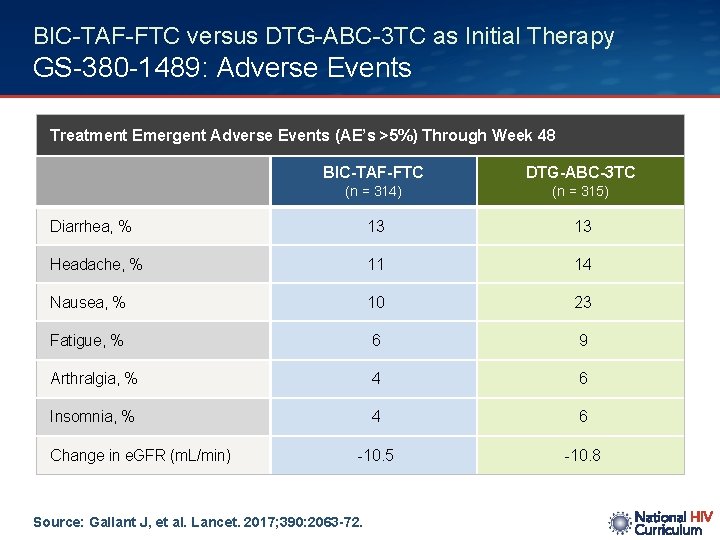
BIC-TAF-FTC versus DTG-ABC-3 TC as Initial Therapy GS-380 -1489: Adverse Events Treatment Emergent Adverse Events (AE’s >5%) Through Week 48 BIC-TAF-FTC DTG-ABC-3 TC (n = 314) (n = 315) Diarrhea, % 13 13 Headache, % 11 14 Nausea, % 10 23 Fatigue, % 6 9 Arthralgia, % 4 6 Insomnia, % 4 6 -10. 5 -10. 8 Change in e. GFR (m. L/min) Source: Gallant J, et al. Lancet. 2017; 390: 2063 -72.
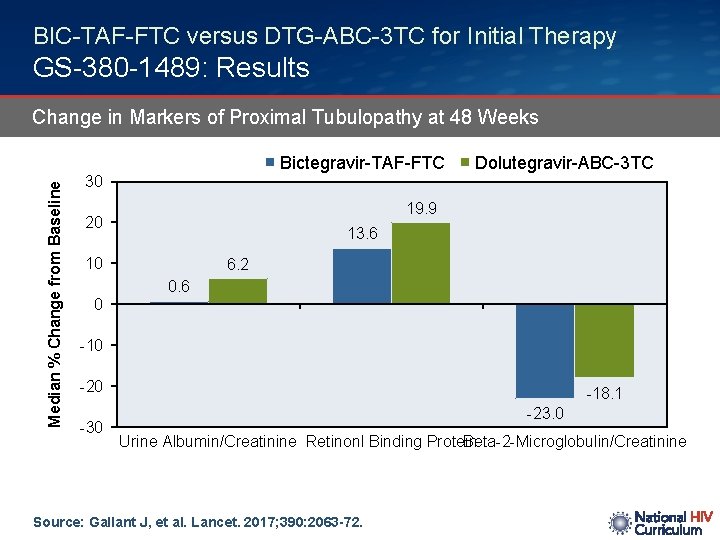
BIC-TAF-FTC versus DTG-ABC-3 TC for Initial Therapy GS-380 -1489: Results Change in Markers of Proximal Tubulopathy at 48 Weeks Median % Change from Baseline Bictegravir-TAF-FTC Dolutegravir-ABC-3 TC 30 19. 9 20 13. 6 10 0 6. 2 0. 6 -10 -20 -30 -18. 1 -23. 0 Urine Albumin/Creatinine Retinonl Binding Protein Beta-2 -Microglobulin/Creatinine Source: Gallant J, et al. Lancet. 2017; 390: 2063 -72.
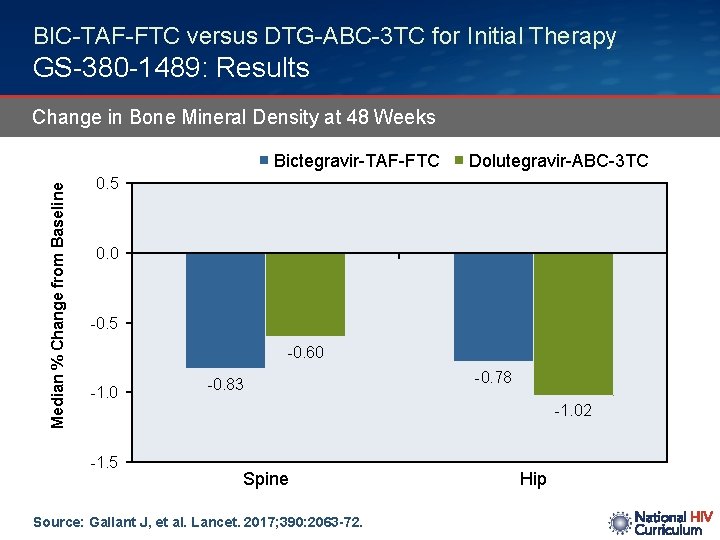
BIC-TAF-FTC versus DTG-ABC-3 TC for Initial Therapy GS-380 -1489: Results Change in Bone Mineral Density at 48 Weeks Median % Change from Baseline Bictegravir-TAF-FTC Dolutegravir-ABC-3 TC 0. 5 0. 0 -0. 5 -0. 60 -1. 0 -0. 83 -0. 78 -1. 02 -1. 5 Spine Source: Gallant J, et al. Lancet. 2017; 390: 2063 -72. Hip
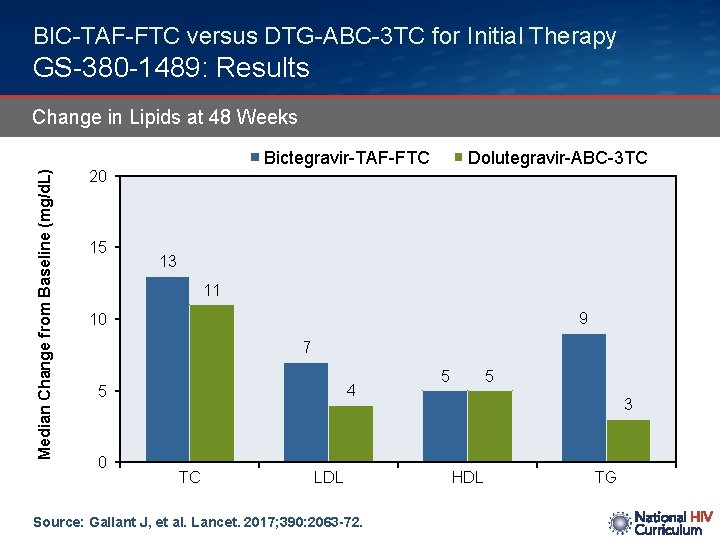
BIC-TAF-FTC versus DTG-ABC-3 TC for Initial Therapy GS-380 -1489: Results Median Change from Baseline (mg/d. L) Change in Lipids at 48 Weeks Bictegravir-TAF-FTC 20 15 Dolutegravir-ABC-3 TC 13 11 9 10 7 4 5 0 TC LDL Source: Gallant J, et al. Lancet. 2017; 390: 2063 -72. 5 5 3 HDL TG
BIC-TAF-FTC versus DTG-ABC-3 TC for Initial Therapy GS-380 -1489: Conclusions Interpretation: “At 48 weeks, coformulated bictegravir, emtricitabine, and tenofovir alafenamide achieved virological suppression in 92% of previously untreated adults and was non-inferior to coformulated dolutegravir, abacavir, and lamivudine, with no treatment-emergent resistance. Bictegravir, emtricitabine, and tenofovir alafenamide was safe and well tolerated with better gastrointestinal tolerability than dolutegravir, abacavir, and lamivudine. Because coformulated bictegravir, emtricitabine, and tenofovir alafenamide does not require HLA B*5701 testing and provides guideline-recommended treatment for individuals co-infected with HIV and hepatitis B, this regimen might lend itself to rapid or same-day initiation of therapy in the clinical setting. ” Source: Gallant J, et al. Lancet. 2017; 390: 2063 -72.

BIC-TAF-FTC vs. DTG + TAF-FTC as Initial Therapy GS-380 -1490: Week 48 Results
BIC-TAF-FTC versus DTG + TAF-FTC as Initial Therapy GS-380 -1490: Design GS-380 -1490: Study Design • Background: Randomized, double-blind, activecontrolled, phase 3 study evaluating the efficacy and safety of bictegravir-tenofovir alafenamideemtricitabine versus dolutegravir plus tenofovir alafenamide-emtricitabine for treatment-naïve adults with HIV • Inclusion Criteria - Age >18 - Antiretroviral-naïve (or ≤ 10 days of treatment) - HIV RNA ≥ 500 copies/m. L - e. GFR ≥ 30 m. L/min • Regimens - Bictegravir-TAF-FTC (50/25/200 mg) - Dolutegravir (50 mg) + TAF-FTC (25/200 mg) Source: Sax PE, et al. Lancet. 2017; 390: 2073 -82. Bictegravir-TAF-FTC (n = 320) Dolutegravir + TAF-FTC (n = 325)
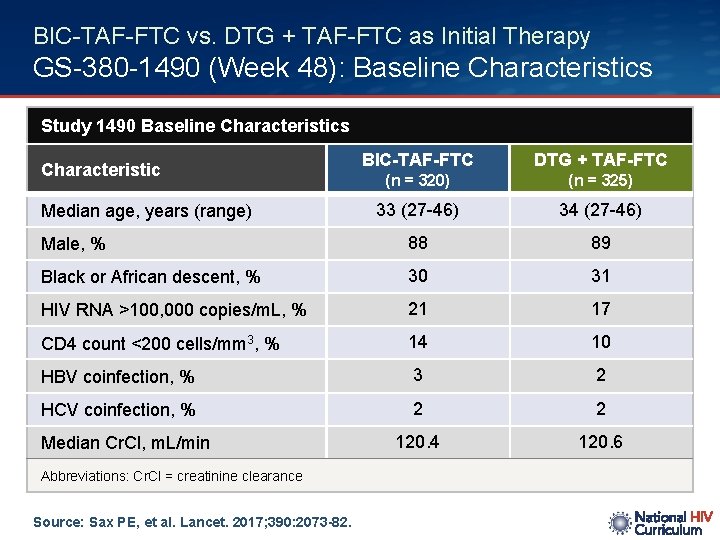
BIC-TAF-FTC vs. DTG + TAF-FTC as Initial Therapy GS-380 -1490 (Week 48): Baseline Characteristics Study 1490 Baseline Characteristics BIC-TAF-FTC DTG + TAF-FTC (n = 320) (n = 325) 33 (27 -46) 34 (27 -46) Male, % 88 89 Black or African descent, % 30 31 HIV RNA >100, 000 copies/m. L, % 21 17 CD 4 count <200 cells/mm 3, % 14 10 HBV coinfection, % 3 2 HCV coinfection, % 2 2 120. 4 120. 6 Characteristic Median age, years (range) Median Cr. Cl, m. L/min Abbreviations: Cr. Cl = creatinine clearance Source: Sax PE, et al. Lancet. 2017; 390: 2073 -82.
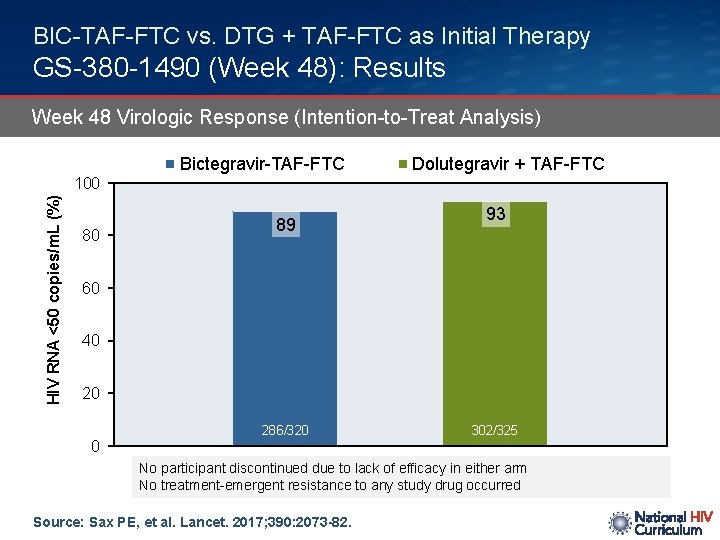
BIC-TAF-FTC vs. DTG + TAF-FTC as Initial Therapy GS-380 -1490 (Week 48): Results Week 48 Virologic Response (Intention-to-Treat Analysis) Bictegravir-TAF-FTC Dolutegravir + TAF-FTC HIV RNA <50 copies/m. L (%) 100 80 89 93 60 40 20 0 286/320 302/325 No participant discontinued due to lack of efficacy in either arm No treatment-emergent resistance to any study drug occurred Source: Sax PE, et al. Lancet. 2017; 390: 2073 -82.
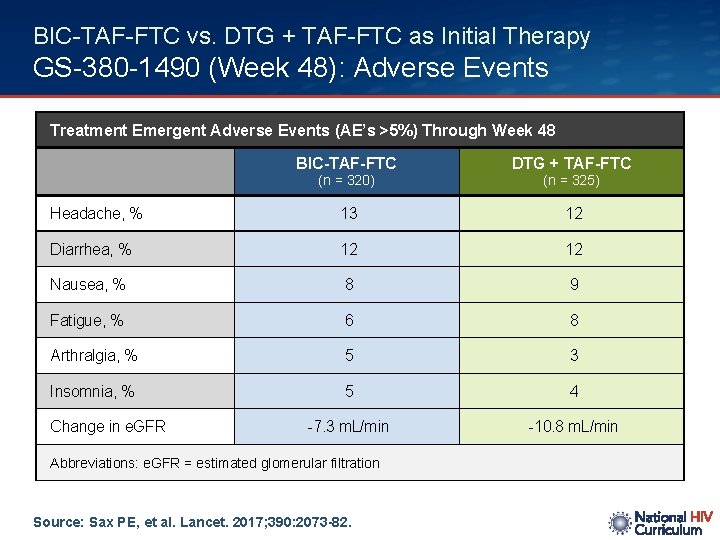
BIC-TAF-FTC vs. DTG + TAF-FTC as Initial Therapy GS-380 -1490 (Week 48): Adverse Events Treatment Emergent Adverse Events (AE’s >5%) Through Week 48 BIC-TAF-FTC DTG + TAF-FTC Headache, % 13 12 Diarrhea, % 12 12 Nausea, % 8 9 Fatigue, % 6 8 Arthralgia, % 5 3 Insomnia, % 5 4 -7. 3 m. L/min -10. 8 m. L/min (n = 320) Change in e. GFR Abbreviations: e. GFR = estimated glomerular filtration Source: Sax PE, et al. Lancet. 2017; 390: 2073 -82. (n = 325)

BIC-TAF-FTC vs. DTG + TAF-FTC as Initial Therapy GS-380 -1490 (Week 48): Conclusions Interpretation: “At 48 weeks, virological suppression with the bictegravir regimen was achieved and was non-inferior to the dolutegravir regimen in previously untreated adults. There was no emergent resistance to either regimen. The fixed-dose combination of bictegravir, emtricitabine, and tenofovir alafenamide was safe and well tolerated compared with the dolutegravir regimen. ” Source: Sax PE, et al. Lancet. 2017; 390: 2073 -82.

BIC-TAF-FTC vs. DTG + TAF-FTC as Initial Therapy GS-380 -1490: Week 96 Results
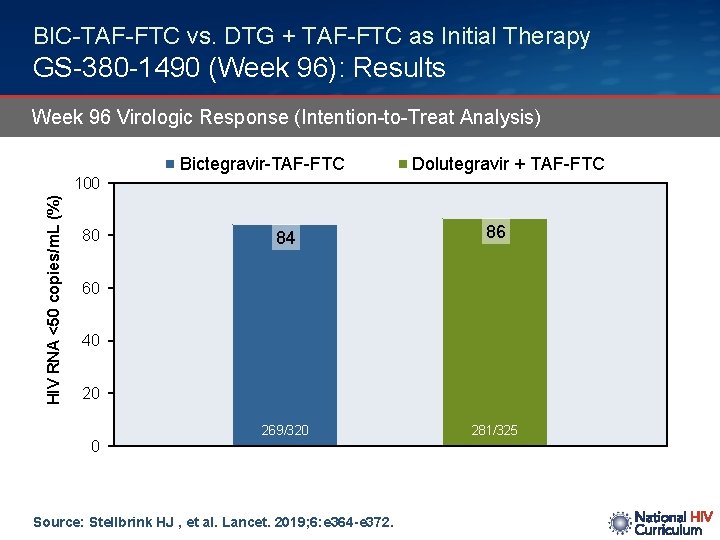
BIC-TAF-FTC vs. DTG + TAF-FTC as Initial Therapy GS-380 -1490 (Week 96): Results Week 96 Virologic Response (Intention-to-Treat Analysis) Bictegravir-TAF-FTC Dolutegravir + TAF-FTC HIV RNA <50 copies/m. L (%) 100 80 84 86 269/320 281/325 60 40 20 0 Source: Stellbrink HJ , et al. Lancet. 2019; 6: e 364 -e 372.

BIC-TAF-FTC vs. DTG + TAF-FTC as Initial Therapy GS-380 -1490 (Week 96): Conclusions Interpretation: “These week 96 data support bictegravir, emtricitabine, and tenofovir alafenamide as a safe, well tolerated, and durable treatment for people living with chronic HIV. ” Source: Stellbrink HJ , et al. Lancet. 2019; 6: e 364 -e 372.

SWITCH STUDIES Bictegravir-Tenofovir alafenamide-Emtricitabine

Switch from DTG-ABC-3 TC to BIC-TAF-FTC in Adults with Virologic Suppression GS-380 -1844
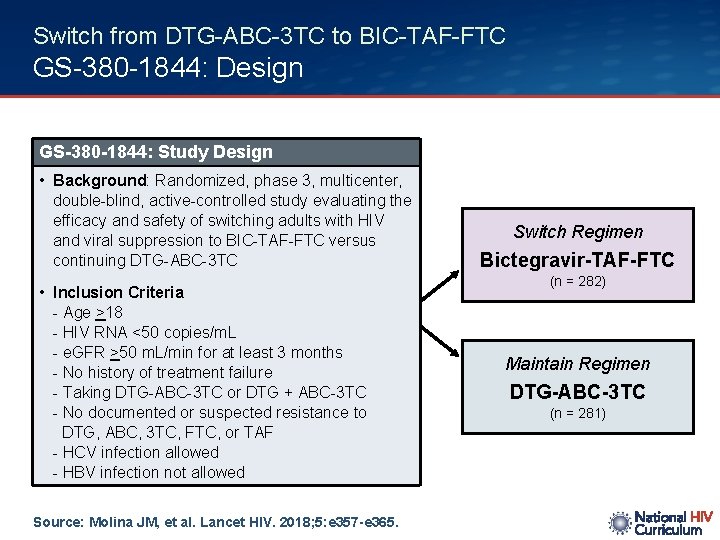
Switch from DTG-ABC-3 TC to BIC-TAF-FTC GS-380 -1844: Design GS-380 -1844: Study Design • Background: Randomized, phase 3, multicenter, double-blind, active-controlled study evaluating the efficacy and safety of switching adults with HIV and viral suppression to BIC-TAF-FTC versus continuing DTG-ABC-3 TC • Inclusion Criteria - Age >18 - HIV RNA <50 copies/m. L - e. GFR >50 m. L/min for at least 3 months - No history of treatment failure - Taking DTG-ABC-3 TC or DTG + ABC-3 TC - No documented or suspected resistance to DTG, ABC, 3 TC, FTC, or TAF - HCV infection allowed - HBV infection not allowed Source: Molina JM, et al. Lancet HIV. 2018; 5: e 357 -e 365. Switch Regimen Bictegravir-TAF-FTC (n = 282) Maintain Regimen DTG-ABC-3 TC (n = 281)
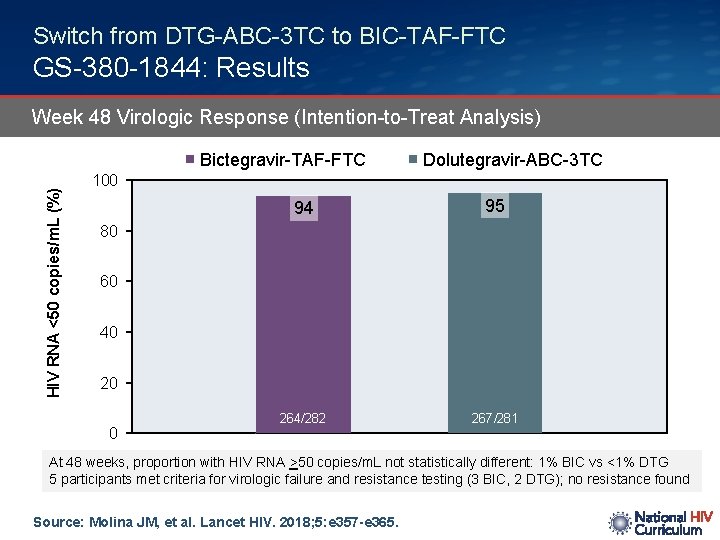
Switch from DTG-ABC-3 TC to BIC-TAF-FTC GS-380 -1844: Results Week 48 Virologic Response (Intention-to-Treat Analysis) HIV RNA <50 copies/m. L (%) Bictegravir-TAF-FTC Dolutegravir-ABC-3 TC 100 94 95 264/282 267/281 80 60 40 20 0 At 48 weeks, proportion with HIV RNA >50 copies/m. L not statistically different: 1% BIC vs <1% DTG 5 participants met criteria for virologic failure and resistance testing (3 BIC, 2 DTG); no resistance found Source: Molina JM, et al. Lancet HIV. 2018; 5: e 357 -e 365.
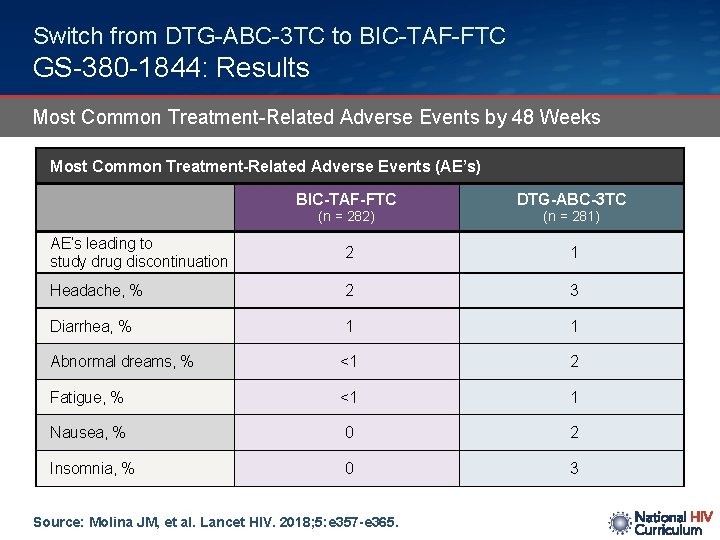
Switch from DTG-ABC-3 TC to BIC-TAF-FTC GS-380 -1844: Results Most Common Treatment-Related Adverse Events by 48 Weeks Most Common Treatment-Related Adverse Events (AE’s) BIC-TAF-FTC DTG-ABC-3 TC AE’s leading to study drug discontinuation 2 1 Headache, % 2 3 Diarrhea, % 1 1 Abnormal dreams, % <1 2 Fatigue, % <1 1 Nausea, % 0 2 Insomnia, % 0 3 (n = 282) Source: Molina JM, et al. Lancet HIV. 2018; 5: e 357 -e 365. (n = 281)
Switch from DTG-ABC-3 TC to BIC-TAF-FTC GS-380 -1844: Conclusions Interpretation: “The fixed-dose combination of bictegravir, emtricitabine, and tenofovir alafenamide might provide a safe and efficacious option for ongoing treatment of HIV-1 infection. ” Source: Molina JM, et al. Lancet HIV. 2018; 5: e 357 -e 365.

Switch from Boosted PI + 2 NRTI’s to BIC-TAF-FTC with Viral Suppression GS-380 -1878
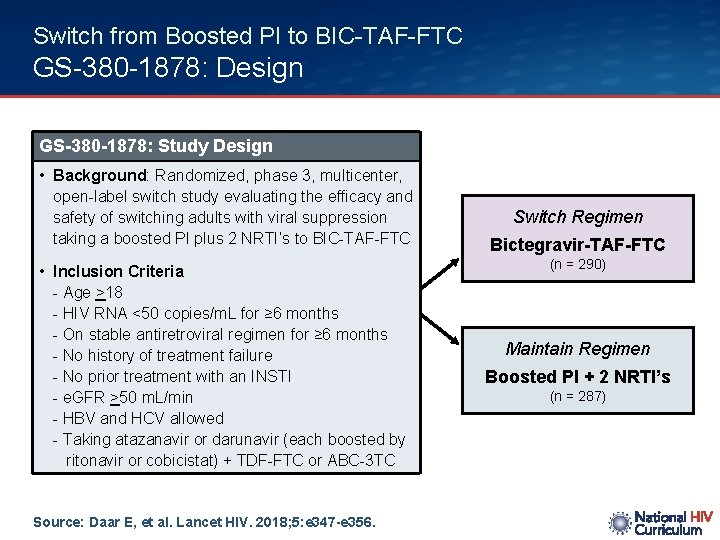
Switch from Boosted PI to BIC-TAF-FTC GS-380 -1878: Design GS-380 -1878: Study Design • Background: Randomized, phase 3, multicenter, open-label switch study evaluating the efficacy and safety of switching adults with viral suppression taking a boosted PI plus 2 NRTI’s to BIC-TAF-FTC • Inclusion Criteria - Age >18 - HIV RNA <50 copies/m. L for ≥ 6 months - On stable antiretroviral regimen for ≥ 6 months - No history of treatment failure - No prior treatment with an INSTI - e. GFR >50 m. L/min - HBV and HCV allowed - Taking atazanavir or darunavir (each boosted by ritonavir or cobicistat) + TDF-FTC or ABC-3 TC Source: Daar E, et al. Lancet HIV. 2018; 5: e 347 -e 356. Switch Regimen Bictegravir-TAF-FTC (n = 290) Maintain Regimen Boosted PI + 2 NRTI’s (n = 287)
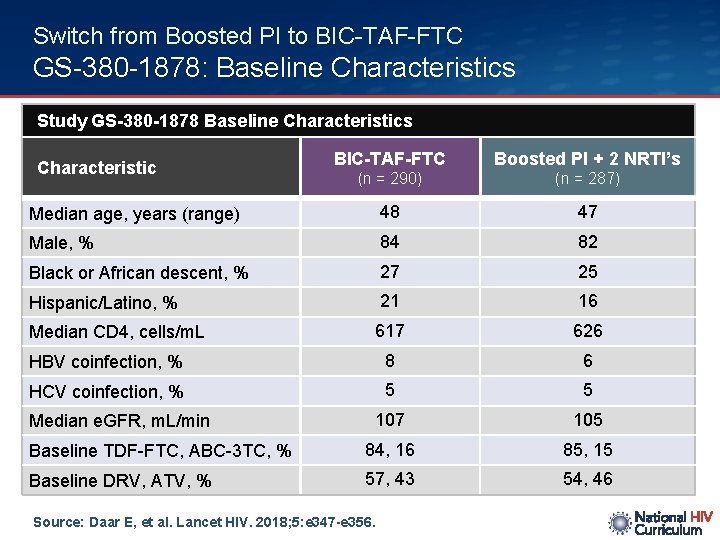
Switch from Boosted PI to BIC-TAF-FTC GS-380 -1878: Baseline Characteristics Study GS-380 -1878 Baseline Characteristics BIC-TAF-FTC Boosted PI + 2 NRTI’s (n = 290) (n = 287) Median age, years (range) 48 47 Male, % 84 82 Black or African descent, % 27 25 Hispanic/Latino, % 21 16 Median CD 4, cells/m. L 617 626 HBV coinfection, % 8 6 HCV coinfection, % 5 5 107 105 Baseline TDF-FTC, ABC-3 TC, % 84, 16 85, 15 Baseline DRV, ATV, % 57, 43 54, 46 Characteristic Median e. GFR, m. L/min Source: Daar E, et al. Lancet HIV. 2018; 5: e 347 -e 356.
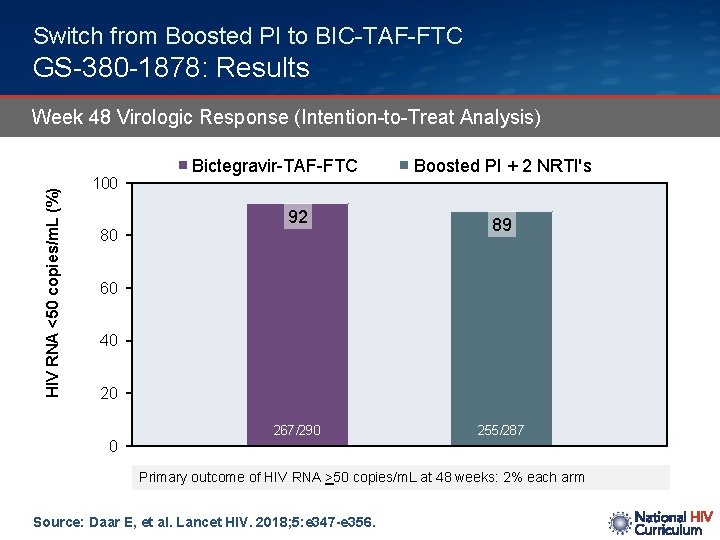
Switch from Boosted PI to BIC-TAF-FTC GS-380 -1878: Results HIV RNA <50 copies/m. L (%) Week 48 Virologic Response (Intention-to-Treat Analysis) 100 Bictegravir-TAF-FTC 92 80 Boosted PI + 2 NRTI's 89 60 40 20 0 267/290 255/287 Primary outcome of HIV RNA >50 copies/m. L at 48 weeks: 2% each arm Source: Daar E, et al. Lancet HIV. 2018; 5: e 347 -e 356.
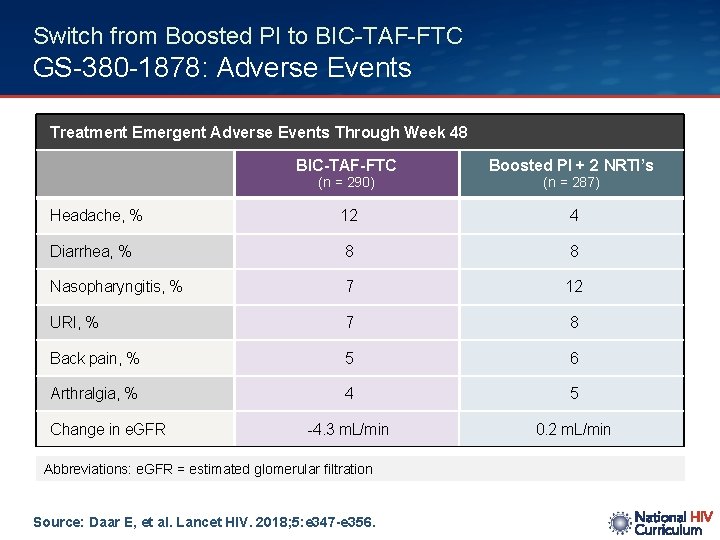
Switch from Boosted PI to BIC-TAF-FTC GS-380 -1878: Adverse Events Treatment Emergent Adverse Events Through Week 48 BIC-TAF-FTC Boosted PI + 2 NRTI’s Headache, % 12 4 Diarrhea, % 8 8 Nasopharyngitis, % 7 12 URI, % 7 8 Back pain, % 5 6 Arthralgia, % 4 5 -4. 3 m. L/min 0. 2 m. L/min (n = 290) Change in e. GFR Abbreviations: e. GFR = estimated glomerular filtration Source: Daar E, et al. Lancet HIV. 2018; 5: e 347 -e 356. (n = 287)
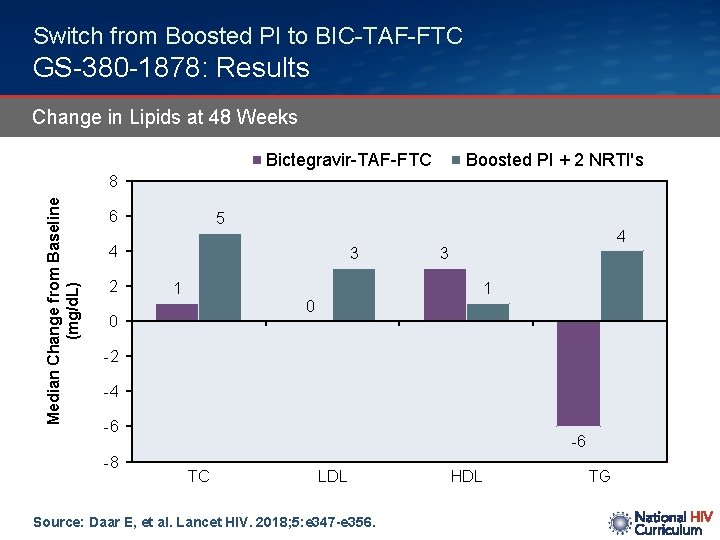
Switch from Boosted PI to BIC-TAF-FTC GS-380 -1878: Results Change in Lipids at 48 Weeks Bictegravir-TAF-FTC Boosted PI + 2 NRTI's Median Change from Baseline (mg/d. L) 8 6 5 4 2 3 1 4 3 1 0 0 -2 -4 -6 -8 -6 TC LDL Source: Daar E, et al. Lancet HIV. 2018; 5: e 347 -e 356. HDL TG
Switch from Boosted PI to BIC-TAF-FTC GS-380 -1878: Conclusions Interpretation: “Fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide might be a safe and efficacious alternative to continued boosted protease inhibitor therapy in adults with HIV-1 infection. ” Source: Daar E, et al. Lancet HIV. 2018; 5: e 347 -e 356.

Switch to BIC-TAF-FTC in Women with Virologic Suppression GS-380 -1961
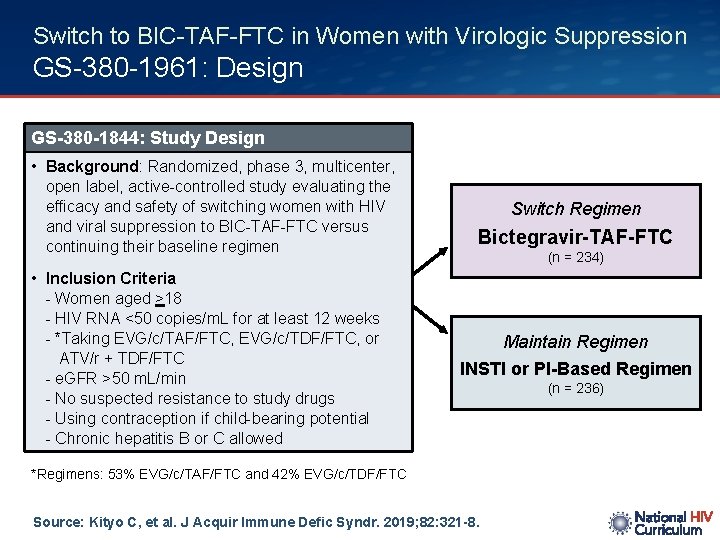
Switch to BIC-TAF-FTC in Women with Virologic Suppression GS-380 -1961: Design GS-380 -1844: Study Design • Background: Randomized, phase 3, multicenter, open label, active-controlled study evaluating the efficacy and safety of switching women with HIV and viral suppression to BIC-TAF-FTC versus continuing their baseline regimen • Inclusion Criteria - Women aged >18 - HIV RNA <50 copies/m. L for at least 12 weeks - *Taking EVG/c/TAF/FTC, EVG/c/TDF/FTC, or ATV/r + TDF/FTC - e. GFR >50 m. L/min - No suspected resistance to study drugs - Using contraception if child-bearing potential - Chronic hepatitis B or C allowed Switch Regimen Bictegravir-TAF-FTC (n = 234) Maintain Regimen INSTI or PI-Based Regimen *Regimens: 53% EVG/c/TAF/FTC and 42% EVG/c/TDF/FTC Source: Kityo C, et al. J Acquir Immune Defic Syndr. 2019; 82: 321 -8. (n = 236)
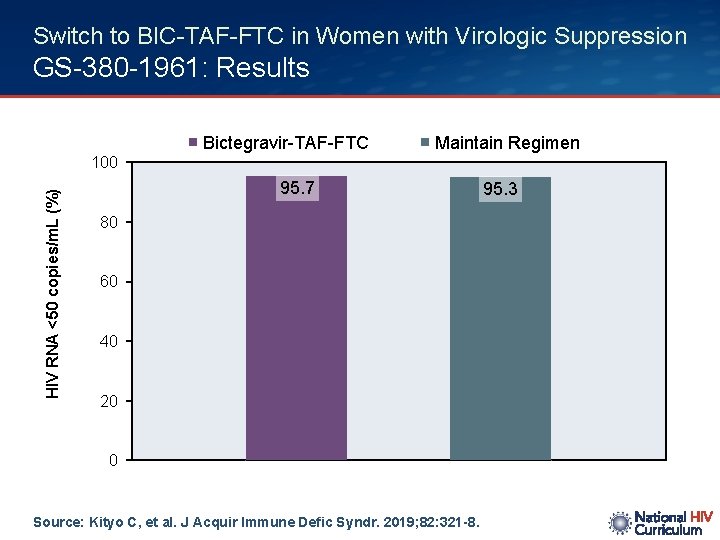
Switch to BIC-TAF-FTC in Women with Virologic Suppression GS-380 -1961: Results Bictegravir-TAF-FTC Maintain Regimen HIV RNA <50 copies/m. L (%) 100 95. 7 80 60 40 20 0 Source: Kityo C, et al. J Acquir Immune Defic Syndr. 2019; 82: 321 -8. 95. 3
Switch to BIC-TAF-FTC in Women with Virologic Suppression GS-380 -1961: Conclusions Interpretation: “Fixed-dose combination bictegraviremtricitabine-tenofovir alafenamide provides a safe and efficacious option for ongoing treatment of HIV in women. This study contributes important data on safety, tolerability, and outcomes of antiretroviral therapy among women living with HIV. ” Source: Kityo C, et al. J Acquir Immune Defic Syndr. 2019; 82: 321 -8.

BIC-TAF-FTC Switch Studies (1844 and 1878) Impact of Archived M 184 V Mutation
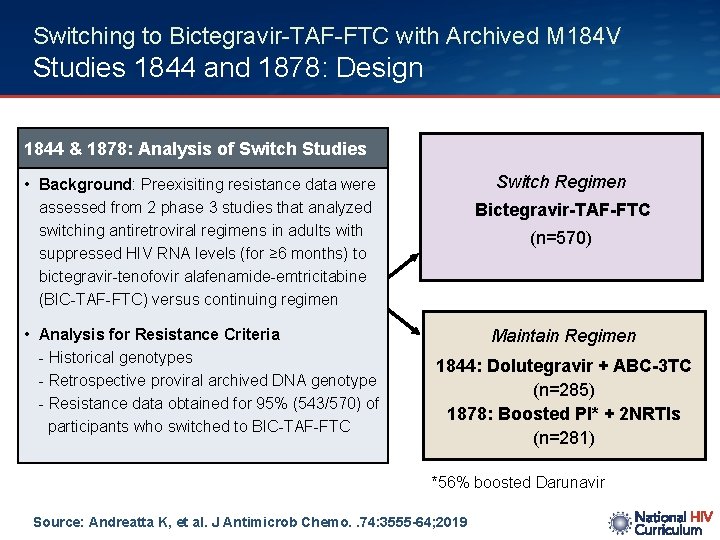
Switching to Bictegravir-TAF-FTC with Archived M 184 V Studies 1844 and 1878: Design 1844 & 1878: Analysis of Switch Studies Switch Regimen • Background: Preexisiting resistance data were assessed from 2 phase 3 studies that analyzed switching antiretroviral regimens in adults with suppressed HIV RNA levels (for ≥ 6 months) to bictegravir-tenofovir alafenamide-emtricitabine (BIC-TAF-FTC) versus continuing regimen • Analysis for Resistance Criteria - Historical genotypes - Retrospective proviral archived DNA genotype - Resistance data obtained for 95% (543/570) of participants who switched to BIC-TAF-FTC Bictegravir-TAF-FTC (n=570) Maintain Regimen 1844: Dolutegravir + ABC-3 TC (n=285) 1878: Boosted PI* + 2 NRTIs (n=281) *56% boosted Darunavir Source: Andreatta K, et al. J Antimicrob Chemo. . 74: 3555 -64; 2019
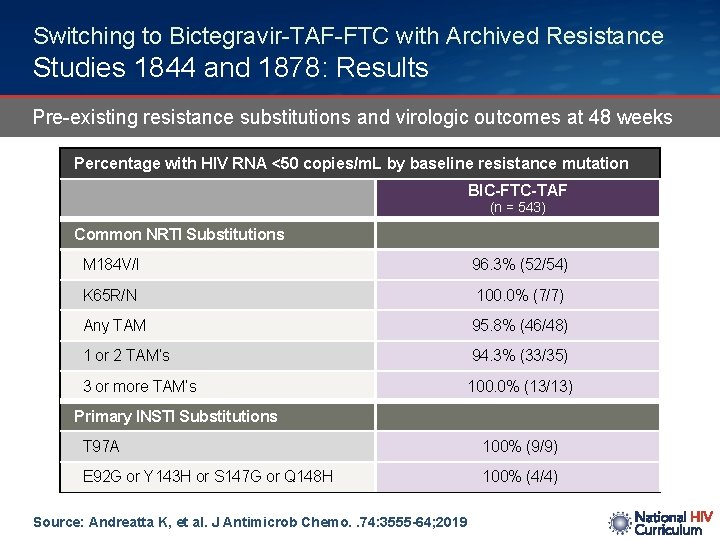
Switching to Bictegravir-TAF-FTC with Archived Resistance Studies 1844 and 1878: Results Pre-existing resistance substitutions and virologic outcomes at 48 weeks Percentage with HIV RNA <50 copies/m. L by baseline resistance mutation BIC-FTC-TAF (n = 543) Common NRTI Substitutions M 184 V/I 96. 3% (52/54) K 65 R/N 100. 0% (7/7) Any TAM 95. 8% (46/48) 1 or 2 TAM’s 94. 3% (33/35) 3 or more TAM’s 100. 0% (13/13) Primary INSTI Substitutions T 97 A 100% (9/9) E 92 G or Y 143 H or S 147 G or Q 148 H 100% (4/4) Source: Andreatta K, et al. J Antimicrob Chemo. . 74: 3555 -64; 2019
Switching to Bictegravir-TAF-FTC with Archived M 184 V Studies 1844 and 1878: Key Points • Baseline M 184 V/I in 10% of switch group (BIC-TAF-FTC) • 96% (52/54) with archived M 184 V had HIV RNA <50 copies/m. L for up to 48 weeks on BIC-TAF-FTC Source: Andreatta K, et al. J Antimicrob Chemo. . 74: 3555 -64; 2019
Switching to Bictegravir-TAF-FTC with Archived M 184 V Studies 1878 and 1844: Conclusions Interpretation: “Pre-existing resistance substitutions, notably M 184 V/I, were unexpectedly common among suppressed participants who switched to BIC/FTC/TAF. High rates of virological suppression were maintained in the overall study population and in those with pre-existing resistance, including M 184 V/I, for up to 48 weeks of BIC/FTC/TAF treatment with no resistance development. These results indicate that BIC/FTC/TAF is an effective treatment option for suppressed patients, including those with evidence of archived NRTI resistance. ” Source: Andreatta K, et al. J Antimicrob Chemo. 2019; pii: dkz 347. [Epub ahead of print]

Bictegravir 10 -day Dose-Ranging Monotherapy GS-141 -1219
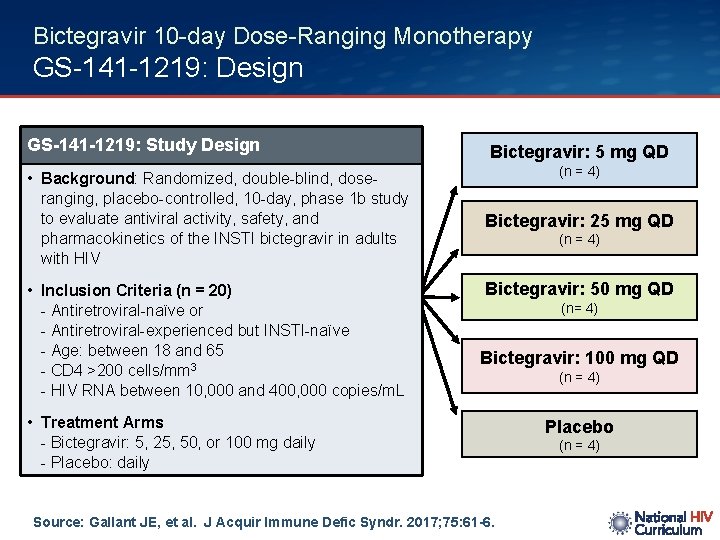
Bictegravir 10 -day Dose-Ranging Monotherapy GS-141 -1219: Design GS-141 -1219: Study Design • Background: Randomized, double-blind, dose- ranging, placebo-controlled, 10 -day, phase 1 b study to evaluate antiviral activity, safety, and pharmacokinetics of the INSTI bictegravir in adults with HIV • Inclusion Criteria (n = 20) - Antiretroviral-naïve or - Antiretroviral-experienced but INSTI-naïve - Age: between 18 and 65 - CD 4 >200 cells/mm 3 - HIV RNA between 10, 000 and 400, 000 copies/m. L Bictegravir: 5 mg QD (n = 4) Bictegravir: 25 mg QD (n = 4) Bictegravir: 50 mg QD (n= 4) Bictegravir: 100 mg QD • Treatment Arms - Bictegravir: 5, 25, 50, or 100 mg daily - Placebo: daily Source: Gallant JE, et al. J Acquir Immune Defic Syndr. 2017; 75: 61 -6. (n = 4) Placebo (n = 4)
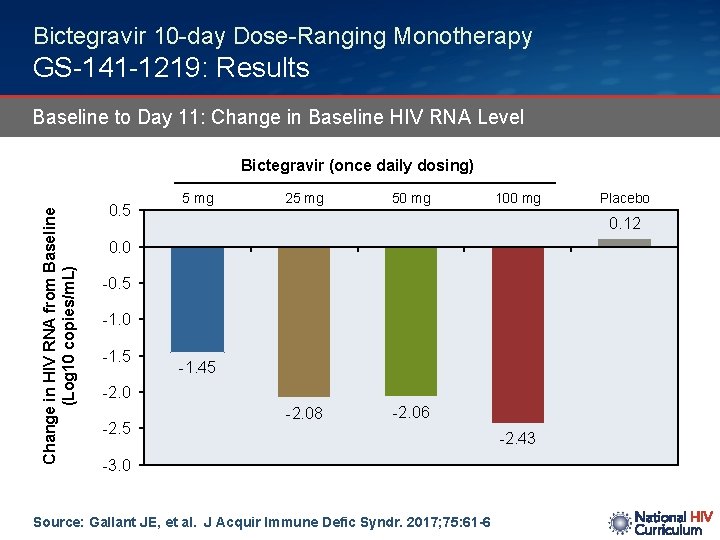
Bictegravir 10 -day Dose-Ranging Monotherapy GS-141 -1219: Results Baseline to Day 11: Change in Baseline HIV RNA Level Change in HIV RNA from Baseline (Log 10 copies/m. L) Bictegravir (once daily dosing) 0. 5 5 mg 25 mg 50 mg 100 mg 0. 12 0. 0 -0. 5 -1. 0 -1. 5 -1. 45 -2. 0 -2. 5 Placebo -2. 08 -2. 06 -3. 0 Source: Gallant JE, et al. J Acquir Immune Defic Syndr. 2017; 75: 61 -6 -2. 43
Bictegravir 10 -day Dose-Ranging Monotherapy GS-141 -1219: Conclusions Interpretation: “Bictegravir is a novel, potent, unboosted integrase strand transfer inhibitor (INSTI) that demonstrated rapid, dose-dependent declines in HIV-1 RNA after 10 days of monotherapy. Bictegravir was well tolerated, and displayed rapid absorption and a half-life supportive of once-daily therapy in HIV-infected subjects. ” Source: Gallant JE, et al. J Acquir Immune Defic Syndr. 2017; 75: 61 -6

Acknowledgment The National HIV Curriculum is an AIDS Education and Training Center (AETC) Program supported by the Health Resources and Services Administration (HRSA) of the U. S. Department of Health and Human Services (HHS) as part of an award totaling $800, 000 with 0% financed with non-governmental sources. This project is led by the University of Washington’s Infectious Diseases Education and Assessment (IDEA) Program. The content in this presentation are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by HRSA, HHS, or the U. S. Government.
- Slides: 57