Betatrace Protein A New Biomarker for CSF Leakage

Beta-trace Protein A New Biomarker for CSF Leakage and Residual Renal Function Dr. Lenard Mueller, June 2017 Unrestricted © Siemens Healthcare Diagnostics Inc. 2017

Beta-trace Protein: Abundance and Biochemistry Beta-trace Protein (BTP) • Also known as prostaglandin D 2 -synthase • Enzyme for catalyzing production of prostaglandin D 2 , which is an endogenous, sleep-promoting substance • Synthesis by arachnoid membrane and choroid plexus • Second-most-abundant protein in CSF • Molecular weight: ~24 k. Da • Free glomerular filtration in kidneys Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 2 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017

Beta-trace Protein: A Marker for Detection of CSF Leakage Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 3 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017

BTP: Occurrence and Concentration BTP is found in: • Cerebrospinal fluid (CSF) • Perilymph (inner ear fluid) • Serum/plasma Concentration: • In serum: • In CSF: • In perilymph: 0. 59± 0. 23 mg/L* 19. 6± 5. 8 mg/L* (about 33 -fold higher) 51. 5± 48. 9 mg/L† (about 100 -fold higher) Only 3% of CSF in serum results in a 100% increase in the BTP serum sample concentration. *Meco C, et al. New guidelines for the reliable diagnostic of cerebrospinal fluid fistula. Otolaryngol Head Neck Surg. 2003 Nov; 129(5): 508 -17. †Michel O, et al. ß-trace protein (prostaglandin D synthase) - a stable and reliable protein in perilymph. GMS Ger Med Sci. 2005; 3: Doc 04. Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 4 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017

CSF Leakage Possible settings for CSF leakage: • Traumatic brain injury (TBI) • Recent head, nose, or ear surgery • Spontaneous leaks Danger of severe infections, including bacterial meningitis, etc. Clinical need: Early detection, noninvasive test Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 5 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017

Detecting CSF Leakage Suspicion of CSF leakage MRI ß 2 -Transferrin Electrophoresis: ~2 hours* *Schnabel C, et al. Comparison of ß 2 -transferrin and ß-trace protein for detection of cerebrospinal fluid in nasal and ear fluids. Clin Chem. 2004; 50(3): 661 -3. Availability of N Latex BTP assay may vary from country to country. N Latex BTP Assay 12 minutes (assay time) Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 6 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
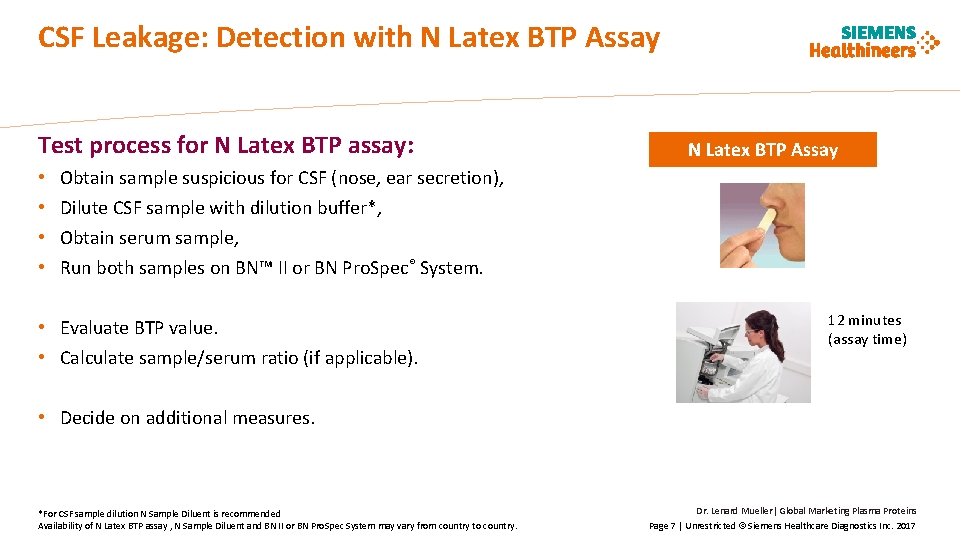
CSF Leakage: Detection with N Latex BTP Assay Test process for N Latex BTP assay: • • N Latex BTP Assay Obtain sample suspicious for CSF (nose, ear secretion), Dilute CSF sample with dilution buffer*, Obtain serum sample, Run both samples on BN™ II or BN Pro. Spec® System. • Evaluate BTP value. • Calculate sample/serum ratio (if applicable). 12 minutes (assay time) • Decide on additional measures. *For CSF sample dilution N Sample Diluent is recommended Availability of N Latex BTP assay , N Sample Diluent and BN II or BN Pro. Spec System may vary from country to country. Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 7 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
CSF Leakage: Clinical Sensitivity of N Latex BTP Assay 2002: Clinical study with 138 samples (oto-/rhinorrhoea) tested for CSF leakage*: No false-positive results compared to intraoperative findings, CT, MRI, and radionuclide cisternography. • Negative predictive value: • Positive predictive value: • Overall accuracy: 0. 971 1 0. 974 *Bachmann G, et al. Predictive values of ß-trace protein (prostaglandin D synthase) by use of lasernephelometry assay for the identification of cerebrospinal fluid. Neurosurgery. 2002 Mar; 50(3). Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 8 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
CSF Leakage: Clinical Sensitivity of N Latex BTP Assay 2016: Clinical study with 233 paired secretion/serum samples tested for CSF leakage led to an improved decision algorithm*: No false-positive results compared to intraoperative findings, CT, MRI, and radionuclide cisternography. • • Sensitivity: Specificity: 98% 96% Positive predictive value: Negative predictive value: 89% 99% *Bernasconi L, Pötzl T, Steuer C, et al. Retrospective validation of a β-trace protein interpretation algorithm for the diagnosis of cerebrospinal fluid leakage. Clin Chem Lab Med. 2017 Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 9 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
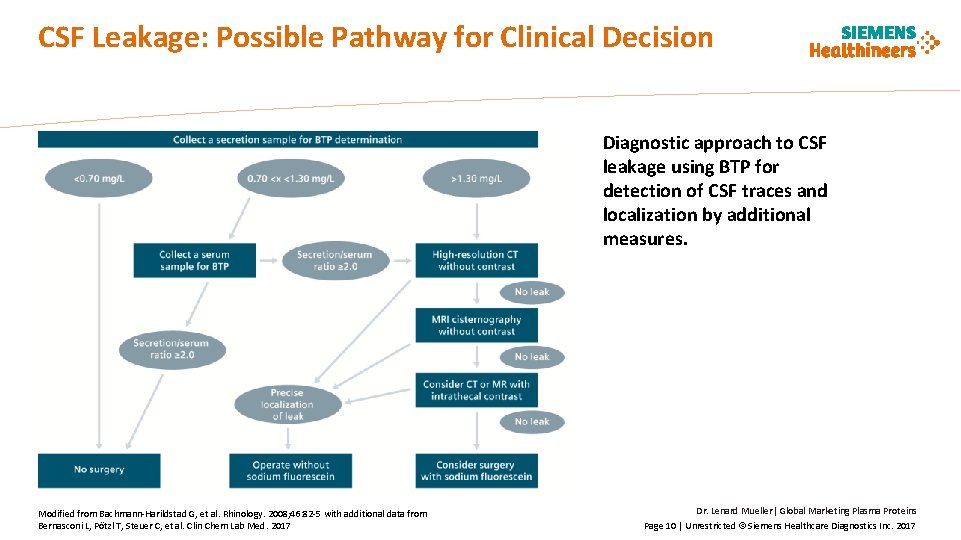
CSF Leakage: Possible Pathway for Clinical Decision Diagnostic approach to CSF leakage using BTP for detection of CSF traces and localization by additional measures. Modified from Bachmann-Harildstad G, et al. Rhinology. 2008; 46: 82 -5 with additional data from Bernasconi L, Pötzl T, Steuer C, et al. Clin Chem Lab Med. 2017 Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 10 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
CSF Leakage: Benefits of N Latex BTP Assay Fast time to result High accuracy Reduced testing costs† More than 10 years’ track record as RUO* product First fully automated immunoassay for BTP †: Morell-Garcia D, et al. , Clin Biochem. 2017; 50: 27 -31. , *RUO: research use only Availability of N Latex BTP assay may vary from country to country. Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 11 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017

Beta-trace Protein: A Marker of Residual Renal Function Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 12 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
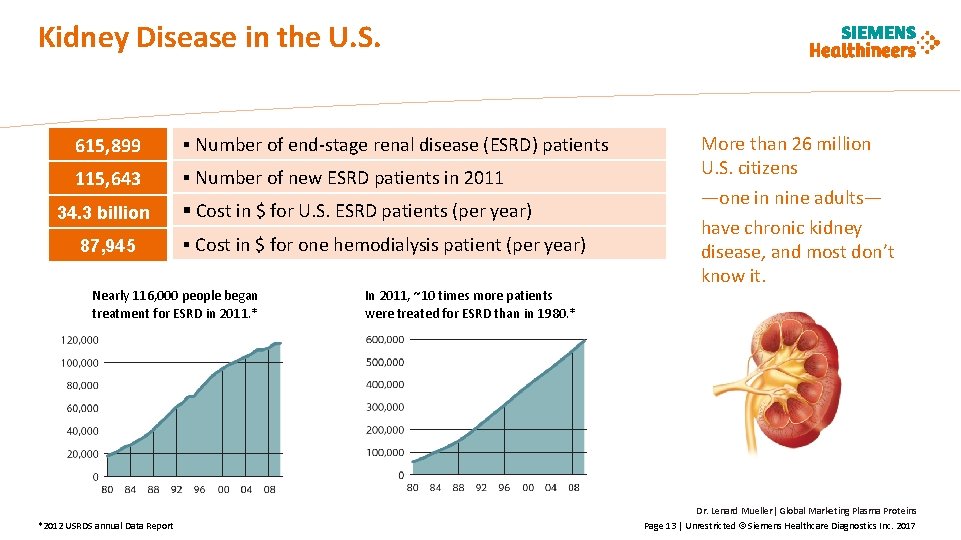
Kidney Disease in the U. S. 615, 899 § Number of end-stage renal disease (ESRD) patients 115, 643 § Number of new ESRD patients in 2011 34. 3 billion 87, 945 § Cost in $ for U. S. ESRD patients (per year) § Cost in $ for one hemodialysis patient (per year) Nearly 116, 000 people began treatment for ESRD in 2011. * *2012 USRDS annual Data Report In 2011, ~10 times more patients were treated for ESRD than in 1980. * More than 26 million U. S. citizens —one in nine adults— have chronic kidney disease, and most don’t know it. Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 13 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
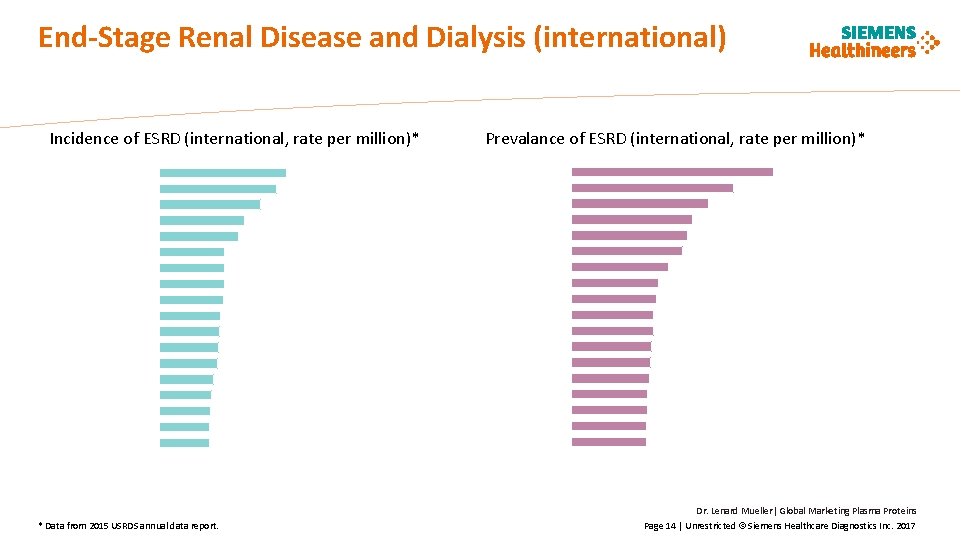
End-Stage Renal Disease and Dialysis (international) Incidence of ESRD (international, rate per million)* Prevalance of ESRD (international, rate per million)* Taiwan Japan United States Singapore Portugal Jalisco (Mexico) Rep. of Korea Chile Belgium, French sp. Belgium, Dutch sp. Hong Kong Canada France Greece Malaysia Israel Uruguay Spain Taiwan Jalisco (Mexico) United States Singapore Japan Malaysia Rep. of Korea Hungary Portugal Thailand Greece Chile Indonesia Czech Republic Belgium, Dutch sp. Belgium, French sp. Brazil Israel - 100 200 300 400 Rate per million population * Data from 2015 USRDS annual data report. 500 0 500 1000 1500 2000 2500 Rate per million population 3000 Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 14 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
Determination of Residual Renal Function (RRF) RRF is an important marker: Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 15 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
Determination of Residual Renal Function (RRF) Despite advances in technology, dialysis can achieve only approximately 15% of native kidney urea clearance and even lower clearance for many other uremic toxins. Native kidney function in RRF is an important marker! dialysis patients, also known as residual renal function (RRF), can contribute significantly to removal of these toxins and help with maintaining fluid balance. As a result, presence of RRF is associated with improved outcomes, better quality of life, and a survival benefit in patients receiving peritoneal dialysis (see CANUSA study*). * Van der Wal WM, et al. Full loss of residual renal function causes higher mortality in dialysis patients: findings from a marginal structural model. Nephrol Dial Transplant. 2011; 26(9): 2978– 83 Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 16 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
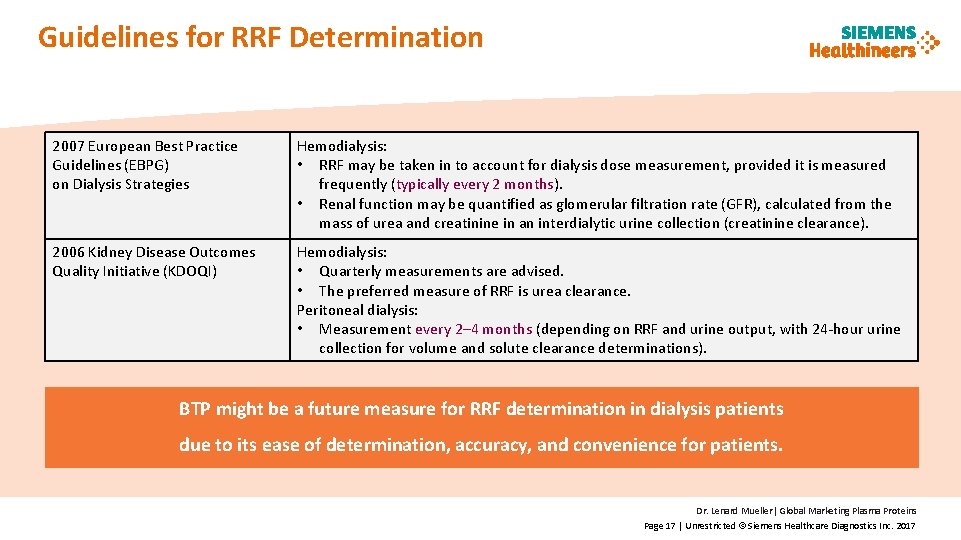
Guidelines for RRF Determination 2007 European Best Practice Guidelines (EBPG) on Dialysis Strategies Hemodialysis: • RRF may be taken in to account for dialysis dose measurement, provided it is measured frequently (typically every 2 months). • Renal function may be quantified as glomerular filtration rate (GFR), calculated from the mass of urea and creatinine in an interdialytic urine collection (creatinine clearance). 2006 Kidney Disease Outcomes Quality Initiative (KDOQI) Hemodialysis: • Quarterly measurements are advised. • The preferred measure of RRF is urea clearance. Peritoneal dialysis: • Measurement every 2– 4 months (depending on RRF and urine output, with 24 -hour urine collection for volume and solute clearance determinations). BTP might be a future measure for RRF determination in dialysis patients due to its ease of determination, accuracy, and convenience for patients. Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 17 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
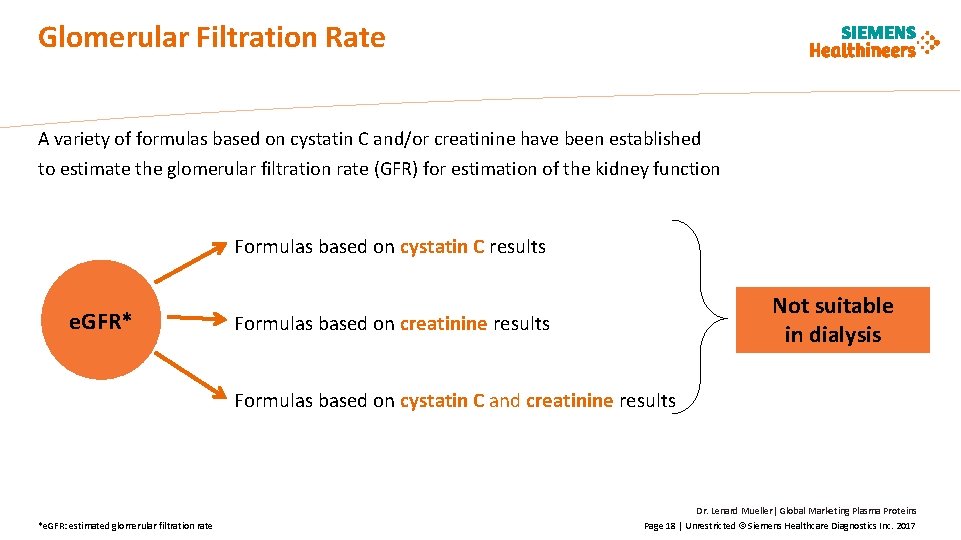
Glomerular Filtration Rate A variety of formulas based on cystatin C and/or creatinine have been established to estimate the glomerular filtration rate (GFR) for estimation of the kidney function Formulas based on cystatin C results e. GFR* Not suitable in dialysis Formulas based on creatinine results Formulas based on cystatin C and creatinine results *e. GFR: estimated glomerular filtration rate Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 18 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
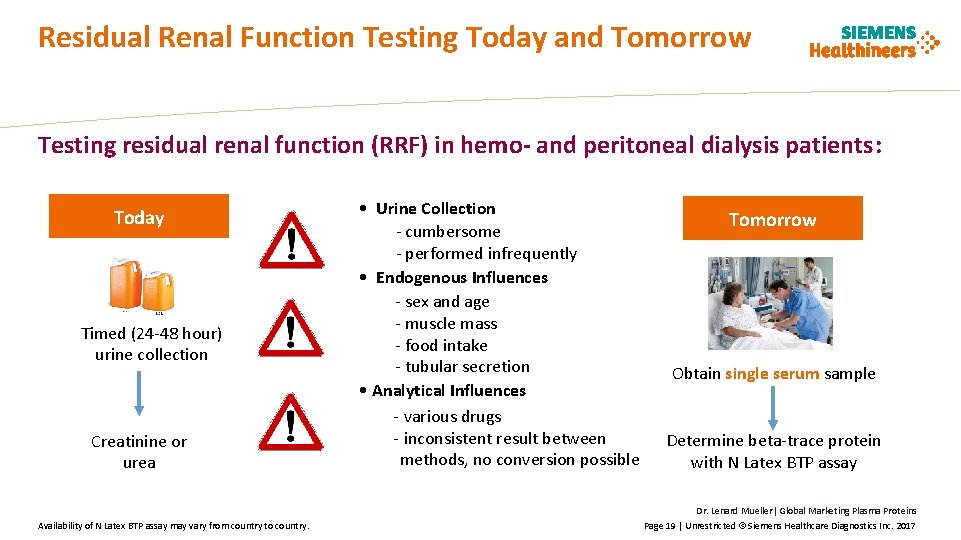
Residual Renal Function Testing Today and Tomorrow Testing residual renal function (RRF) in hemo- and peritoneal dialysis patients: Today Timed (24 -48 hour) urine collection Creatinine or urea Availability of N Latex BTP assay may vary from country to country. • Urine Collection - cumbersome - performed infrequently • Endogenous Influences - sex and age - muscle mass - food intake - tubular secretion • Analytical Influences - various drugs - inconsistent result between methods, no conversion possible Tomorrow Obtain single serum sample Determine beta-trace protein with N Latex BTP assay Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 19 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
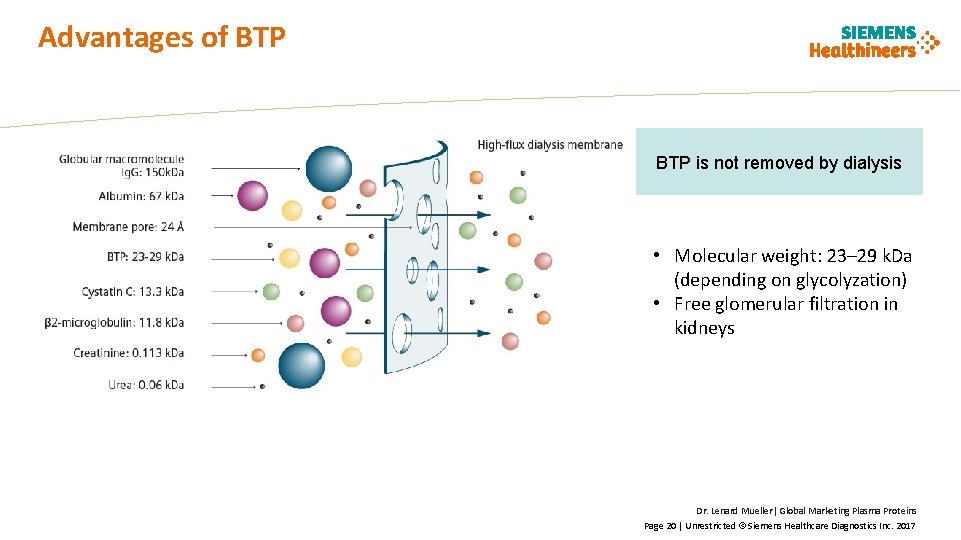
Advantages of BTP is not removed by dialysis • Molecular weight: 23– 29 k. Da (depending on glycolyzation) • Free glomerular filtration in kidneys Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 20 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
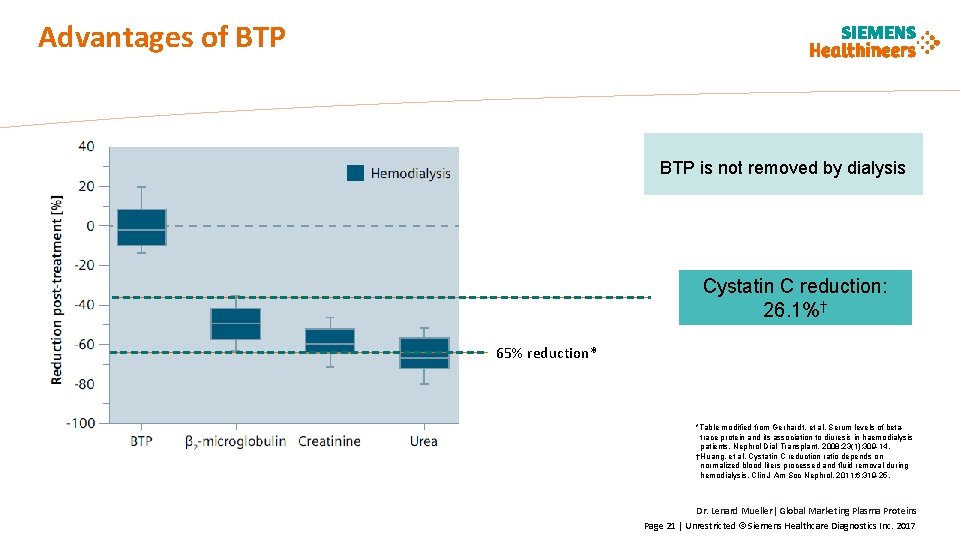
Advantages of BTP is not removed by dialysis Cystatin C reduction: 26. 1%† 65% reduction* *Table modified from Gerhardt, et al. Serum levels of betatrace protein and its association to diuresis in haemodialysis patients. Nephrol Dial Transplant. 2008; 23(1): 309 -14. †Huang, et al. Cystatin C reduction ratio depends on normalized blood liters processed and fluid removal during hemodialysis. Clin J Am Soc Nephrol. 2011; 6: 319 -25. Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 21 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
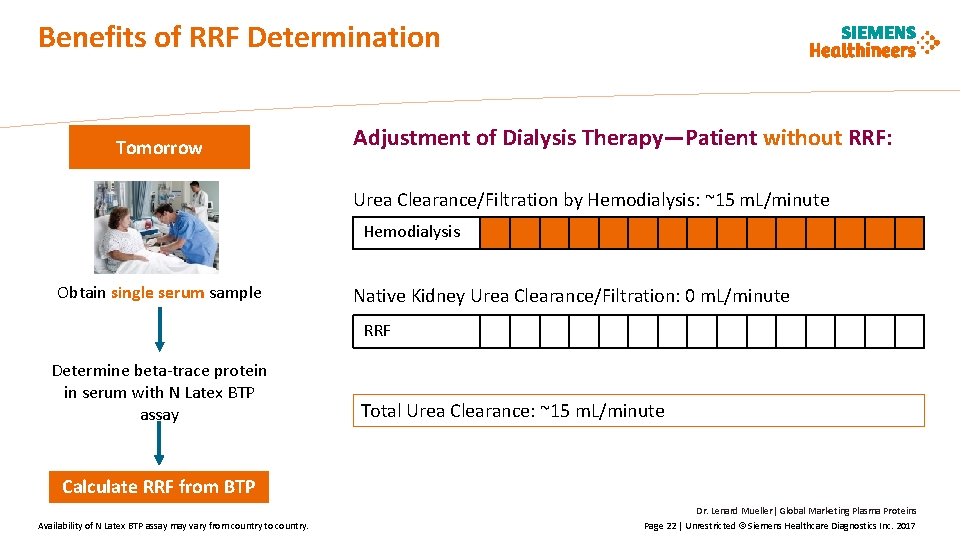
Benefits of RRF Determination Tomorrow Adjustment of Dialysis Therapy—Patient without RRF: Urea Clearance/Filtration by Hemodialysis: ~15 m. L/minute Hemodialysis Obtain single serum sample Native Kidney Urea Clearance/Filtration: 0 m. L/minute RRF Determine beta-trace protein in serum with N Latex BTP assay Total Urea Clearance: ~15 m. L/minute Calculate RRF from BTP Availability of N Latex BTP assay may vary from country to country. Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 22 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
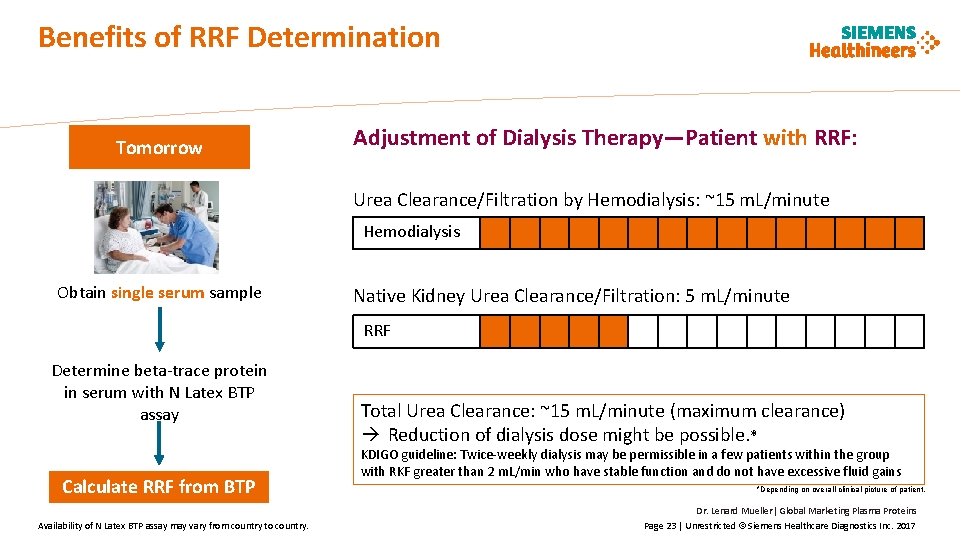
Benefits of RRF Determination Tomorrow Adjustment of Dialysis Therapy—Patient with RRF: Urea Clearance/Filtration by Hemodialysis: ~15 m. L/minute Hemodialysis Obtain single serum sample Native Kidney Urea Clearance/Filtration: 5 m. L/minute RRF Determine beta-trace protein in serum with N Latex BTP assay Calculate RRF from BTP Availability of N Latex BTP assay may vary from country to country. Total Urea Clearance: ~15 m. L/minute (maximum clearance) Reduction of dialysis dose might be possible. * KDIGO guideline: Twice-weekly dialysis may be permissible in a few patients within the group with RKF greater than 2 m. L/min who have stable function and do not have excessive fluid gains *Depending on overall clinical picture of patient. Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 23 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
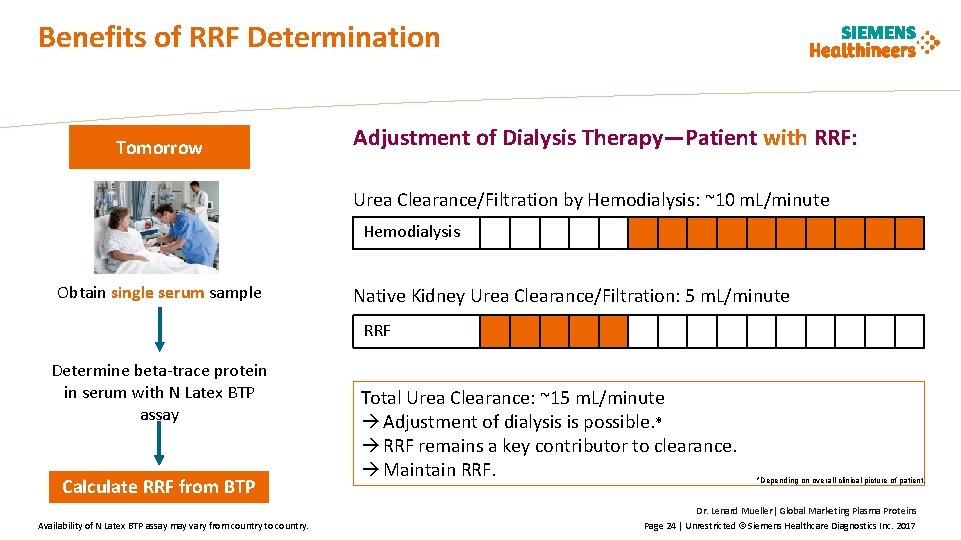
Benefits of RRF Determination Tomorrow Adjustment of Dialysis Therapy—Patient with RRF: Urea Clearance/Filtration by Hemodialysis: ~10 m. L/minute Hemodialysis Obtain single serum sample Native Kidney Urea Clearance/Filtration: 5 m. L/minute RRF Determine beta-trace protein in serum with N Latex BTP assay Calculate RRF from BTP Availability of N Latex BTP assay may vary from country to country. Total Urea Clearance: ~15 m. L/minute Adjustment of dialysis is possible. * RRF remains a key contributor to clearance. Maintain RRF. *Depending on overall clinical picture of patient. Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 24 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
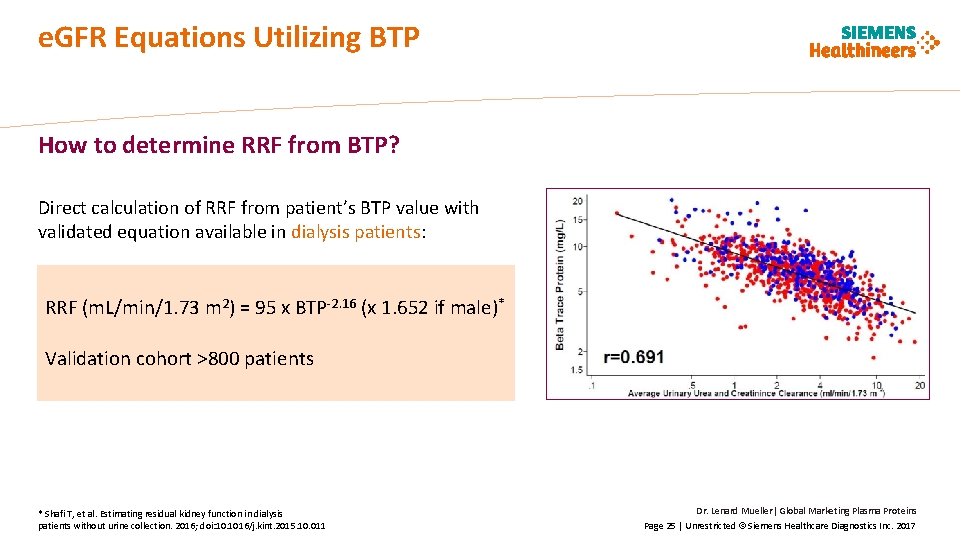
e. GFR Equations Utilizing BTP How to determine RRF from BTP? Direct calculation of RRF from patient’s BTP value with validated equation available in dialysis patients: RRF (m. L/min/1. 73 m 2) = 95 x BTP-2. 16 (x 1. 652 if male)* Validation cohort >800 patients * Shafi T, et al. Estimating residual kidney function in dialysis patients without urine collection. 2016; doi: 10. 1016/j. kint. 2015. 10. 011 Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 25 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
e. GFR Equations Utilizing BTP Measurement of BTP has also been shown to improve e. GFR calcluations in special clinical settings e. GFRBTP is also valuable for patient populations in which cystatin C is of limited value, such as transplant recipients (due to the influence of glucocorticoids on cystatin C levels). e. GFR = 112. 1 x BTP-0. 662 x urea-0. 280 (x 0. 880 if the patient is female)* BTP = mg/L; urea = mmol/L; e. GFR = m. L/min/1. 73 m 2 * White CA, et al. A novel equation to estimate glomerular filtration rate using beta-trace protein. Clin Chem. 2007; 53: 1965 -8. e. GFRBTP can be used in pediatric populations: e. GFR = 10(1. 902 + (0. 9515 x LOG(1/BTP))) ‡ “In summary, the BTP formula proposed here performed significantly better than the Schwartz formula. ” ‡ ‡ Benlamri A, et al. Development of a beta-trace protein based formula for estimation of glomerular filtration rate. Pediatr Nephrol. 2010; 25: 485 -90. Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 26 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
BTP Quick Facts Utilizing BTP in patients with chronic kidney disease and end-stage renal disease BTP reference range (healthy individuals): ≤ 0. 70 mg/L (N Latex BTP IFU) • • BTP values in dialysis patients are approx. 15 -fold higher (compared to healthy individuals). 1 BTP values above 8. 2 mg/L indicate almost complete loss of kidney function. 2 An increase of the BTP level above 8. 8 mg/L is associated with a 63% higher mortality. 3 In pediatric patients, an equation using BTP showed significantly improved accuracy over the wellestablished Schwartz formula. 4 Availability of N Latex BTP assay may vary from country to country. 1. 2. 3. 4. Ruiner CE, et al. Beta trace protein in patients ondergoing peritoneal dialysis. Nephrol. Dial. Transplant. 2015; 30(suppl 3): iii 268. Gerhardt T, et al. Serum levels of beta-trace protein and its association to diuresis in haemodialysis patients. Nephrol Dial Transplant. 2008. 23: 309 -14. Shafi T, et al. Serum β-trace protein and risk of mortality in Incident hemodialysis patients. Clin J Am Soc Nephrol. 2012; 7: 1435– 5. Benlamri A, et al. Development of a beta-trace protein based formula for estimation of glomerular filtration rate. Pediatr Nephrol. 2010; 25(3): 485 -90. Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 27 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
BTP for Assessment of RRF in Dialysis Patients Measuring RRF with beta-trace protein: • Allows estimation and monitoring of RRF with a single serum test (instead of 24– 48 hour urine collection) • Delivers precise results, as BTP is not removed during dialysis • Simplifies design of clinical trials (e. g. , for development and/or monitoring of new drugs) Precise estimation of RRF supports decisions on: • Dialysis modality (peritoneal dialysis vs. hemodialysis) • Dose and frequency of dialysis sessions • Patient diet (food intake) Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 28 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017

Beta-trace Protein Two Clinical Needs Fast and accurate detection of CSF leakage Easy and reliable determination of RRF One Marker Availability of N Latex BTP assay may vary from country to country. Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 29 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
Thank You! Dr. Lenard Mueller Marketing Manager Global Marketing Plasma Proteins Emil-von-Behring-Str. 76 35041 Marburg P +49 (6421) 39 - 4180 F +49 (6421) 39 - 6691 E lenard. mueller@siemens-healthineers. com BN Pro. Spec, BN II and all associated marks are trademarks of Siemens Healthcare Diagnostics Inc. , or its affiliates. Product availability may vary from country to country and is subject to varying regulatory requirements. Please contact your local Siemens Healthineers representative for availability. Dr. Lenard Mueller| Global Marketing Plasma Proteins Page 30 | Unrestricted © Siemens Healthcare Diagnostics Inc. 2017

Now’s our time to inspire the future of healthcare together Engineering success. Pioneering healthcare. Unrestricted © Siemens Healthcare Diagnostics Inc. 2017
- Slides: 31