Betacell glucose sensing glucoreceptors and glucose metabolism Keimyung

Beta-cell glucose sensing: glucoreceptors and glucose metabolism Keimyung University School of Medicine Song, Dae-Kyu

There is no conflict of interest

Three essential elements for glucose-stimulated insulin secretion : glucose metabolism, electrical excitability, extracellular Ca 2+ Henquin, 2000

Two pathways of glucose-stimulated insulin secretion : triggering and amplifying pathway (KATP channel-dependent and -independent pathway) Henquin, 2000
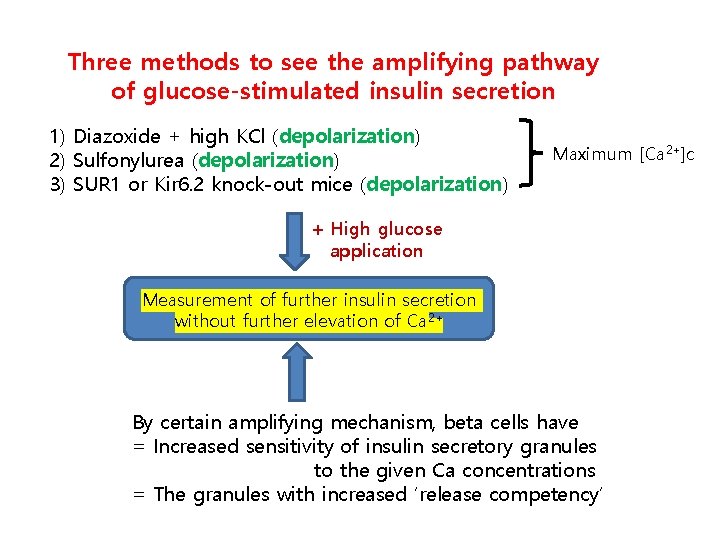
Three methods to see the amplifying pathway of glucose-stimulated insulin secretion 1) Diazoxide + high KCl (depolarization) 2) Sulfonylurea (depolarization) 3) SUR 1 or Kir 6. 2 knock-out mice (depolarization) Maximum [Ca 2+]c + High glucose application Measurement of further insulin secretion without further elevation of Ca 2+ By certain amplifying mechanism, beta cells have = Increased sensitivity of insulin secretory granules to the given Ca concentrations = The granules with increased ‘release competency’
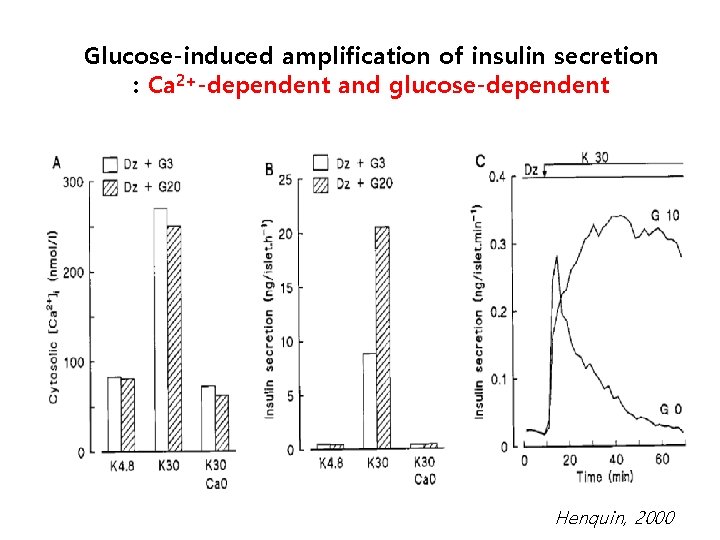
Glucose-induced amplification of insulin secretion : Ca 2+-dependent and glucose-dependent Henquin, 2000
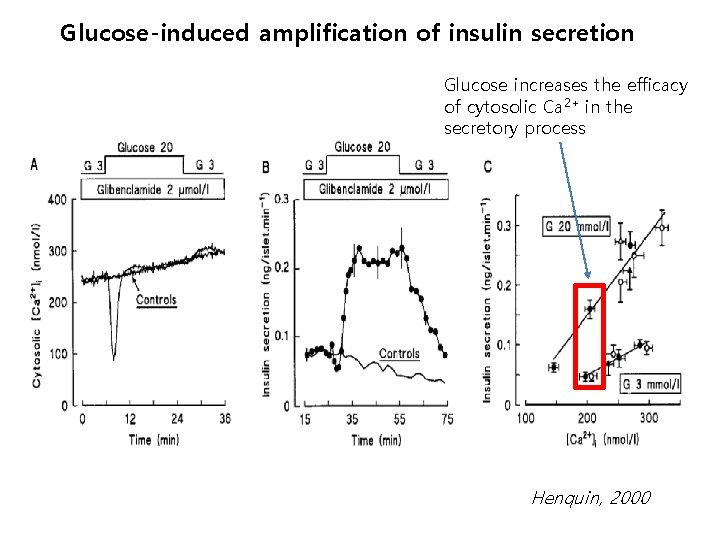
Glucose-induced amplification of insulin secretion Glucose increases the efficacy of cytosolic Ca 2+ in the secretory process Henquin, 2000

Characteristics of glucose-stimulated amplifying pathway High concentration of Ca 2+-dependent : Silent as long as [Ca 2+]i has not been raised by the triggering pathway; otherwise, until glucose has not reached its threshold concentrations to secrete insulin Serves to optimize the secretory response not only to glucose but also to nonglucose stimuli Impaired in type 2 diabetes in animal models and probably humans Novel drugs that correct a deficient amplifying pathway would be useful to restore adequate insulin secretion in type 2 diabetic patients, besides acting on KATP channels and the triggering signal
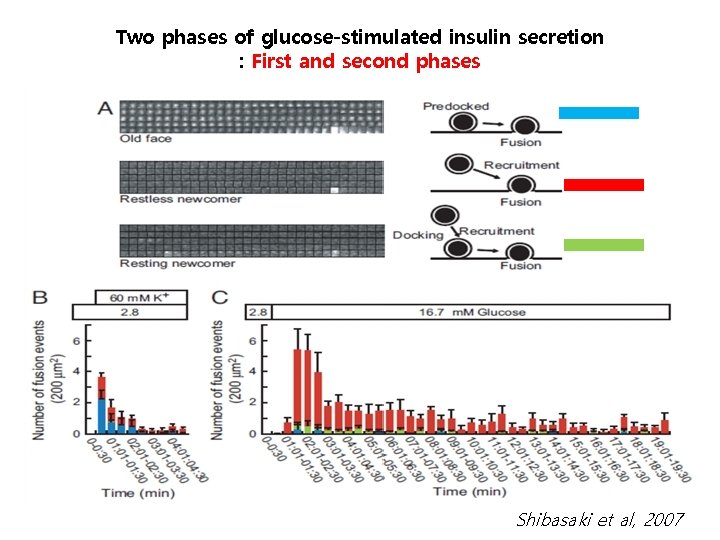
Two phases of glucose-stimulated insulin secretion : First and second phases Shibasaki et al, 2007
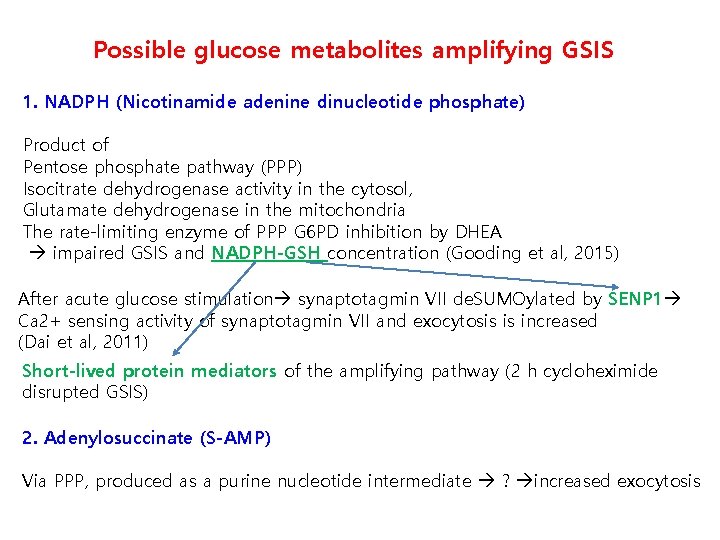
Possible glucose metabolites amplifying GSIS 1. NADPH (Nicotinamide adenine dinucleotide phosphate) Product of Pentose phosphate pathway (PPP) Isocitrate dehydrogenase activity in the cytosol, Glutamate dehydrogenase in the mitochondria The rate-limiting enzyme of PPP G 6 PD inhibition by DHEA impaired GSIS and NADPH-GSH concentration (Gooding et al, 2015) After acute glucose stimulation synaptotagmin VII de. SUMOylated by SENP 1 Ca 2+ sensing activity of synaptotagmin VII and exocytosis is increased (Dai et al, 2011) Short-lived protein mediators of the amplifying pathway (2 h cycloheximide disrupted GSIS) 2. Adenylosuccinate (S-AMP) Via PPP, produced as a purine nucleotide intermediate ? increased exocytosis

3. Glycolysis and the shuttles for NADH delivery to mitochondria Pyruvate generation and influx into mitochondria required for amplification (Patterson et al, 2014) for ATP, GTP and NADPH M-A shuttle and glycerol phosphate shuttle both required for triggering and amplifying pathway (NADH delivery into mitochondria for ATP generation) (Eto, 1999) 4. Lipid signaling : Stimulation of beta cells with glucose alone various FFA including monoacylglycerol (MAG) increase MAG by lipase inhibition insulin secretion increase : direct binding to Munc 13 or GPCR (GPR 40, 41, 43, 119 PLC/PKD) F-actin remodeling (Mugabo et al, 2017)

5. Small GTPases and cytoskeletal signaling Glucose entering ? ? (metabolism? ) (the Src-family kinase Yes small GTPase Cdc 42 kinase Pak 1 GTPase Rac 1) (Raf MEK Erk) Cytoskeletal remodeling (Kepner et al, 2011) 6. Controversial regulatory pathways in glucose-induced amplification - ATP itself lowering vesicular p. H increased priming of the granules - Glucose-induced AC (ADCY 5) activation (metabolism? ), not through GPCR : c. AMP local elevation and oscillation is required in normal GSIS in human islets (Hodson et al, 2014) - Glucose and amino acids GSIS amplification via their plasmalemmal receptors (T 1 R 1, T 1 R 2, T 1 R 3)
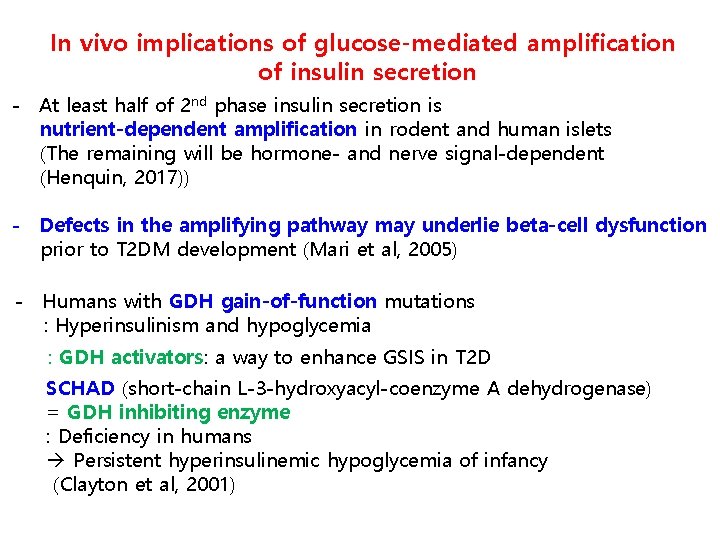
In vivo implications of glucose-mediated amplification of insulin secretion - At least half of 2 nd phase insulin secretion is nutrient-dependent amplification in rodent and human islets (The remaining will be hormone- and nerve signal-dependent (Henquin, 2017)) - Defects in the amplifying pathway may underlie beta-cell dysfunction prior to T 2 DM development (Mari et al, 2005) - Humans with GDH gain-of-function mutations : Hyperinsulinism and hypoglycemia : GDH activators: a way to enhance GSIS in T 2 D SCHAD (short-chain L-3 -hydroxyacyl-coenzyme A dehydrogenase) = GDH inhibiting enzyme : Deficiency in humans Persistent hyperinsulinemic hypoglycemia of infancy (Clayton et al, 2001)
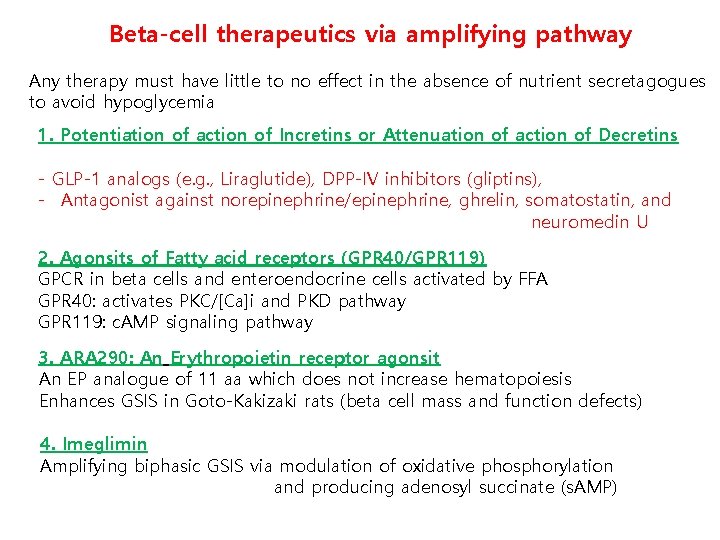
Beta-cell therapeutics via amplifying pathway Any therapy must have little to no effect in the absence of nutrient secretagogues to avoid hypoglycemia 1. Potentiation of action of Incretins or Attenuation of action of Decretins - GLP-1 analogs (e. g. , Liraglutide), DPP-IV inhibitors (gliptins), - Antagonist against norepinephrine/epinephrine, ghrelin, somatostatin, and neuromedin U 2. Agonsits of Fatty acid receptors (GPR 40/GPR 119) GPCR in beta cells and enteroendocrine cells activated by FFA GPR 40: activates PKC/[Ca]i and PKD pathway GPR 119: c. AMP signaling pathway 3. ARA 290: An Erythropoietin receptor agonsit An EP analogue of 11 aa which does not increase hematopoiesis Enhances GSIS in Goto-Kakizaki rats (beta cell mass and function defects) 4. Imeglimin Amplifying biphasic GSIS via modulation of oxidative phosphorylation and producing adenosyl succinate (s. AMP)
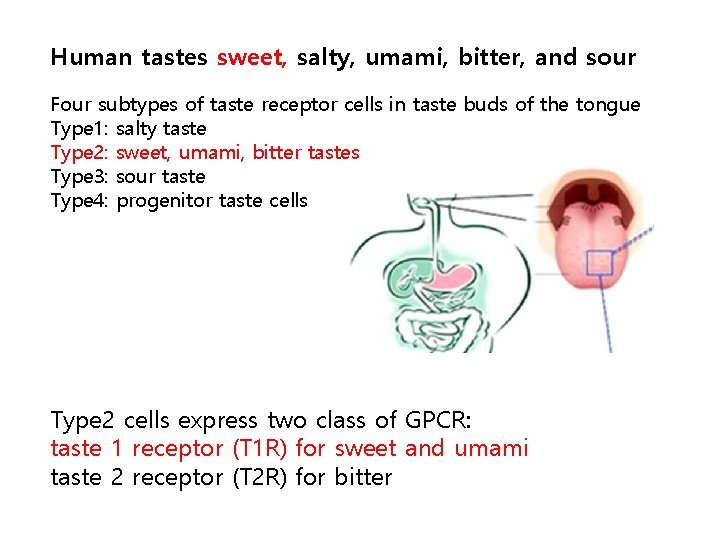
Human tastes sweet, salty, umami, bitter, and sour Four subtypes of taste receptor cells in taste buds of the tongue Type 1: salty taste Type 2: sweet, umami, bitter tastes Type 3: sour taste Type 4: progenitor taste cells Type 2 cells express two class of GPCR: taste 1 receptor (T 1 R) for sweet and umami taste 2 receptor (T 2 R) for bitter
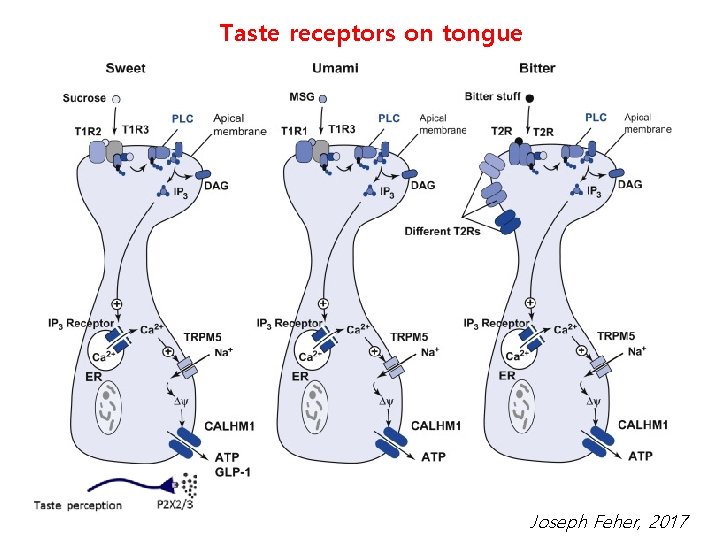
Taste receptors on tongue Joseph Feher, 2017
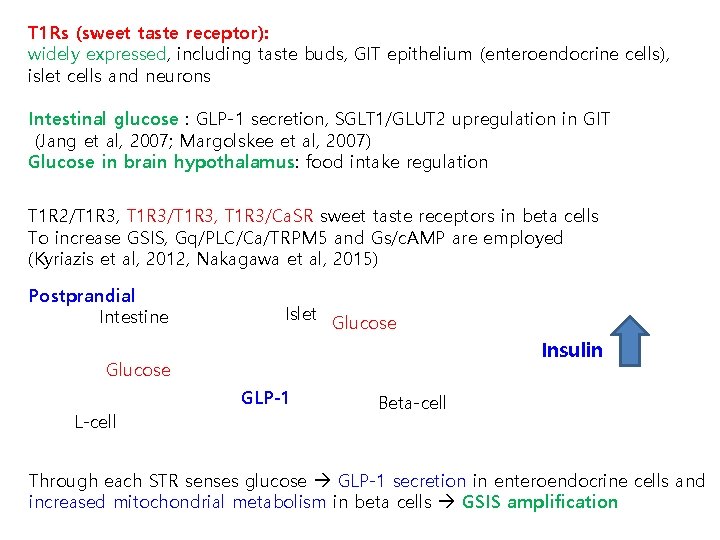
T 1 Rs (sweet taste receptor): widely expressed, including taste buds, GIT epithelium (enteroendocrine cells), islet cells and neurons Intestinal glucose : GLP-1 secretion, SGLT 1/GLUT 2 upregulation in GIT (Jang et al, 2007; Margolskee et al, 2007) Glucose in brain hypothalamus: food intake regulation T 1 R 2/T 1 R 3, T 1 R 3/Ca. SR sweet taste receptors in beta cells To increase GSIS, Gq/PLC/Ca/TRPM 5 and Gs/c. AMP are employed (Kyriazis et al, 2012, Nakagawa et al, 2015) Postprandial Intestine Islet Glucose Insulin Glucose GLP-1 L-cell Beta-cell Through each STR senses glucose GLP-1 secretion in enteroendocrine cells and increased mitochondrial metabolism in beta cells GSIS amplification
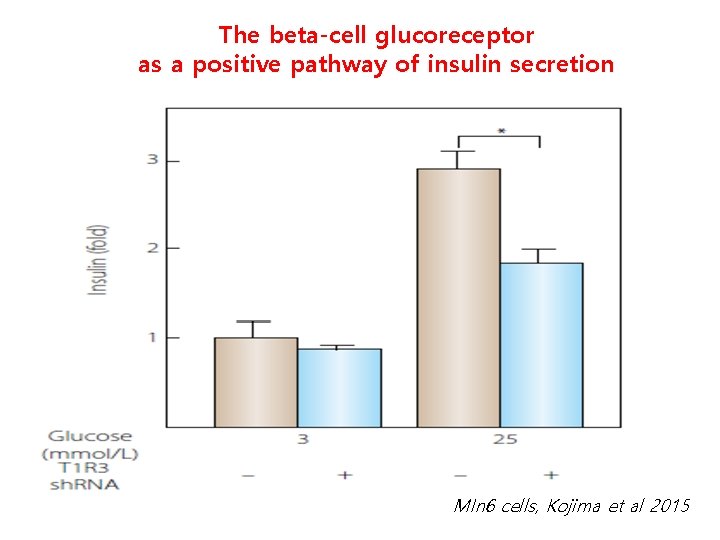
The beta-cell glucoreceptor as a positive pathway of insulin secretion MIn 6 cells, Kojima et al 2015
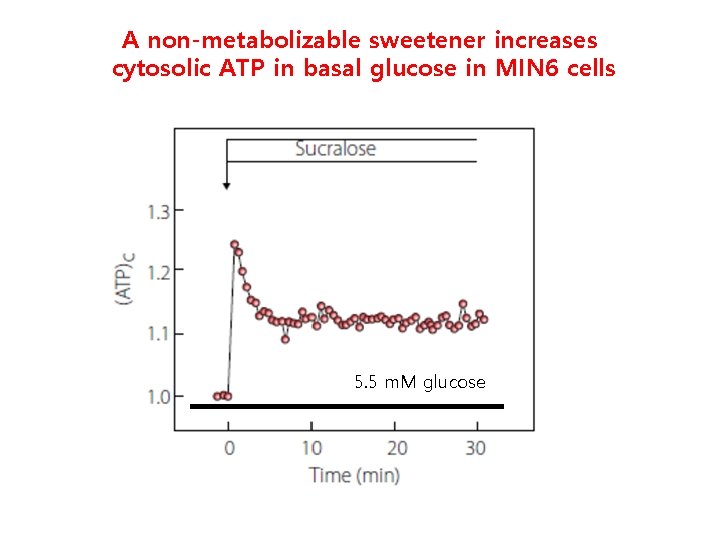
A non-metabolizable sweetener increases cytosolic ATP in basal glucose in MIN 6 cells 5. 5 m. M glucose
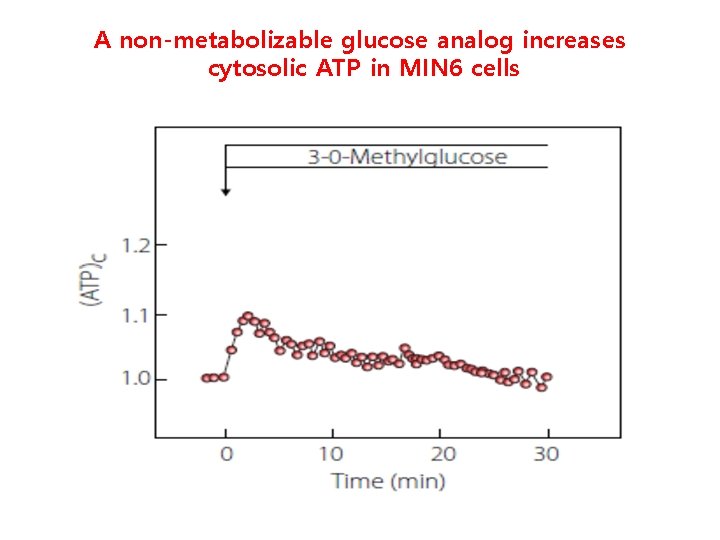
A non-metabolizable glucose analog increases cytosolic ATP in MIN 6 cells
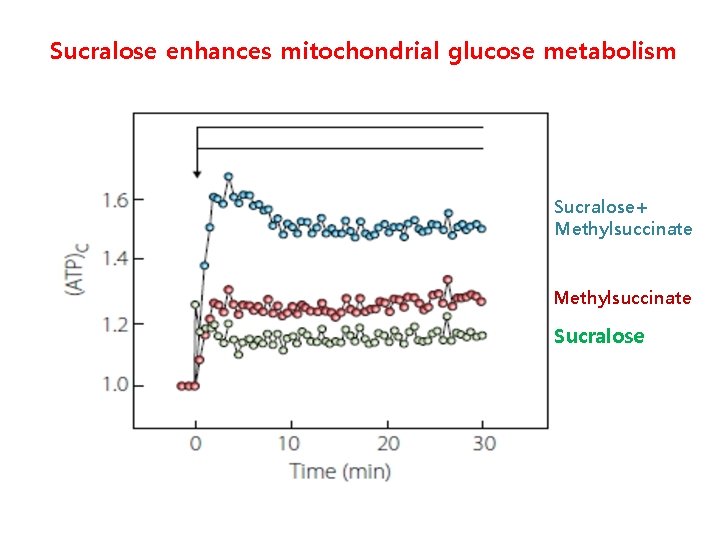
Sucralose enhances mitochondrial glucose metabolism Sucralose+ Methylsuccinate Sucralose

Glucoreceptor KO MIN 6 cells impairs glucose-stimulated ATP generation 25 m. M glucose T 1 R 3(-) MIN 6
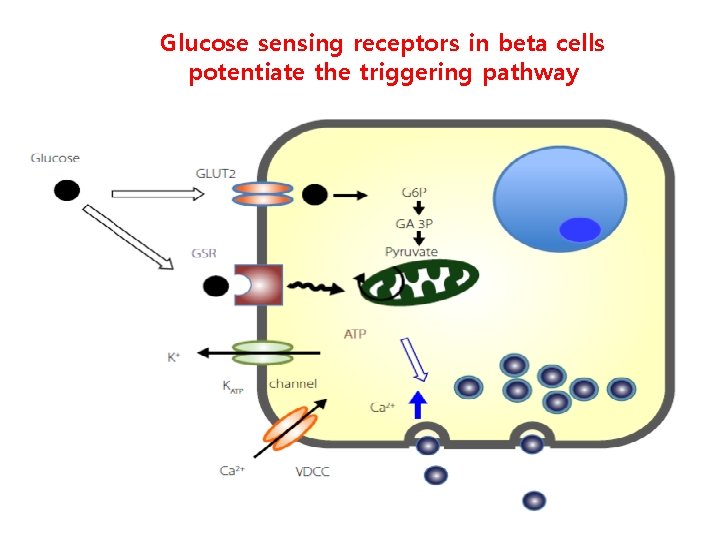
Glucose sensing receptors in beta cells potentiate the triggering pathway
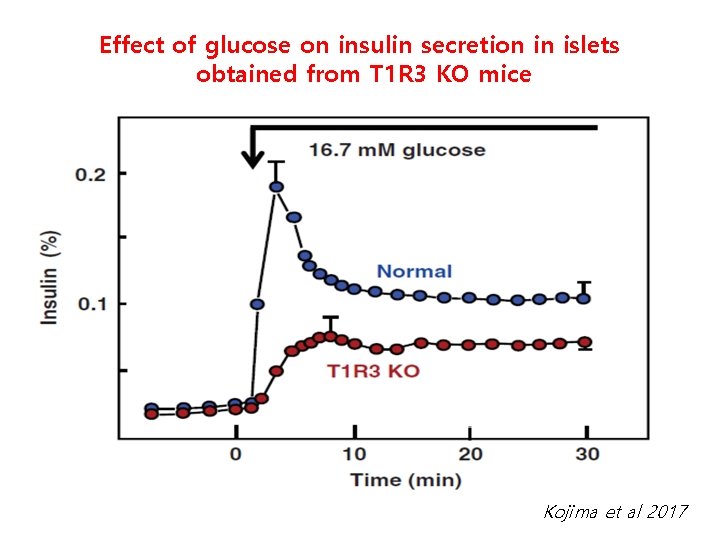
Effect of glucose on insulin secretion in islets obtained from T 1 R 3 KO mice Kojima et al 2017
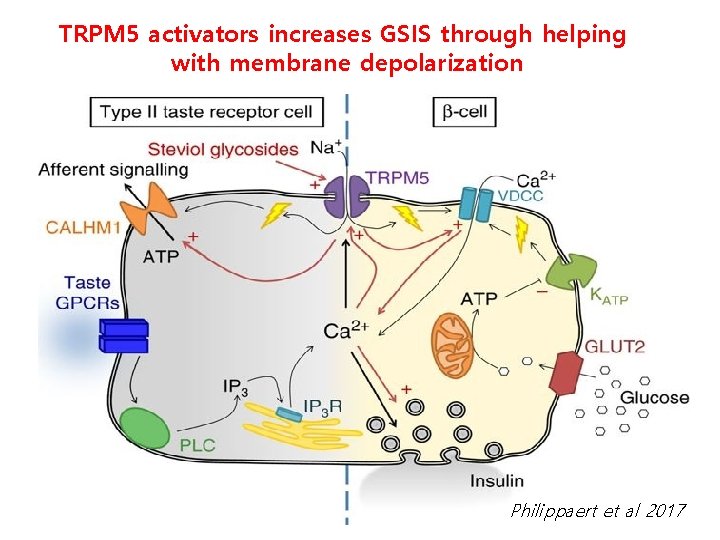
TRPM 5 activators increases GSIS through helping with membrane depolarization Philippaert et al 2017
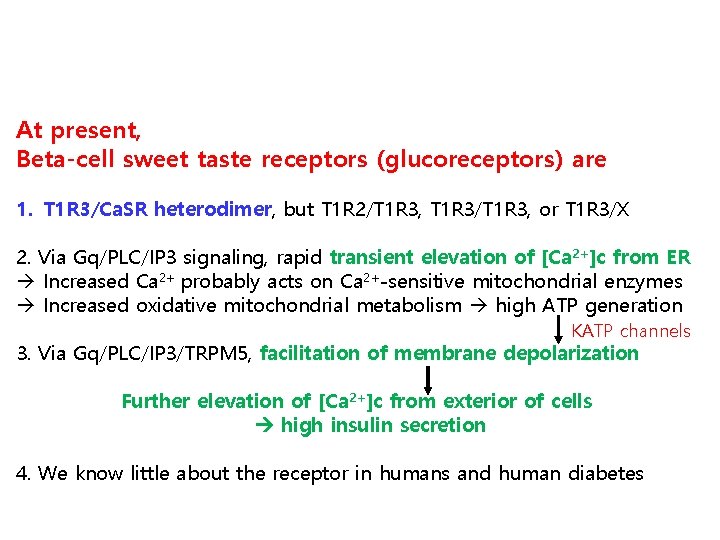
At present, Beta-cell sweet taste receptors (glucoreceptors) are 1. T 1 R 3/Ca. SR heterodimer, but T 1 R 2/T 1 R 3, T 1 R 3/T 1 R 3, or T 1 R 3/X 2. Via Gq/PLC/IP 3 signaling, rapid transient elevation of [Ca 2+]c from ER Increased Ca 2+ probably acts on Ca 2+-sensitive mitochondrial enzymes Increased oxidative mitochondrial metabolism high ATP generation KATP channels 3. Via Gq/PLC/IP 3/TRPM 5, facilitation of membrane depolarization Further elevation of [Ca 2+]c from exterior of cells high insulin secretion 4. We know little about the receptor in humans and human diabetes

Acknowledgements Keimyung University School of Medicine Dr. Park, Jae-Hyung Thank you for your attention!
- Slides: 27