Best Practice Diabetes Drug Management Secrets2014 Loss of

Best Practice Diabetes Drug Management Secrets-2014 Loss of Eyesight Diet/Exercise/Lows /Kidneys/Nerve/ED /Depression Foot Amputation By Sharon A. Watts DNP, RN-BC, CDE

Disclaimers • I have no affiliations with drug companies • I have no affiliations with any industry • I do believe decisions about drugs should be based on evidence, cost to society and individual patient lifestyle & benefit vs. risk

Objectives • Identify common prescribing rules for diabetes drugs. • Select diabetes therapies for several case study presentations based on best practice prescribing knowledge.

Fact or Fiction? New Patient -65 year old DM x 12 years, LDL-145, A 1 c 11%, B/P 162/85, microalbumin/creatinine ratio 200 The Most Important Thing I can do for my patient with diabetes today if I only have time for one change today it should be to lower his high A 1 c? 4
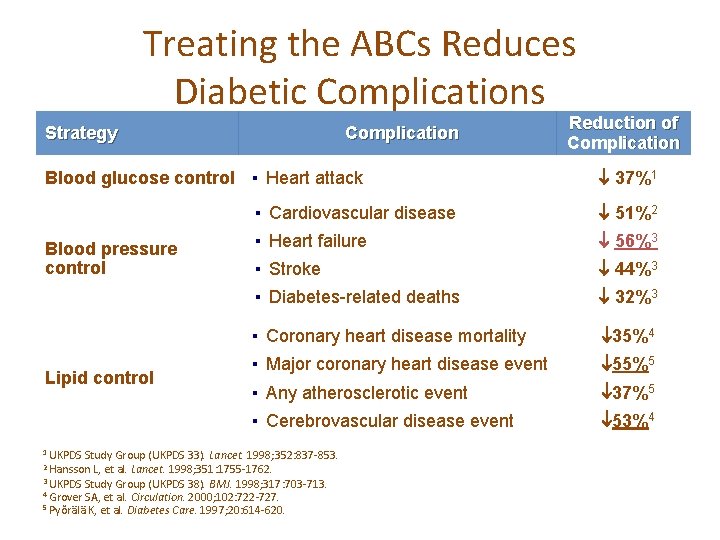
Treating the ABCs Reduces Diabetic Complications Strategy Complication Blood glucose control ▪ Heart attack Blood pressure control Lipid control 37%1 ▪ Cardiovascular disease 51%2 ▪ Heart failure 56%3 ▪ Stroke 44%3 ▪ Diabetes-related deaths 32%3 ▪ Coronary heart disease mortality 35%4 ▪ Major coronary heart disease event 55%5 ▪ Any atherosclerotic event 37%5 ▪ Cerebrovascular disease event 53%4 1 UKPDS Study Group (UKPDS 33). Lancet. 1998; 352: 837 -853. 2 Hansson L, et al. Lancet. 1998; 351: 1755 -1762. 3 UKPDS Study Group (UKPDS 38). BMJ. 1998; 317: 703 -713. 4 Grover SA, et al. Circulation. 2000; 102: 722 -727. 5 Pyŏrälä K, et al. Diabetes Reduction of Complication Care. 1997; 20: 614 -620.
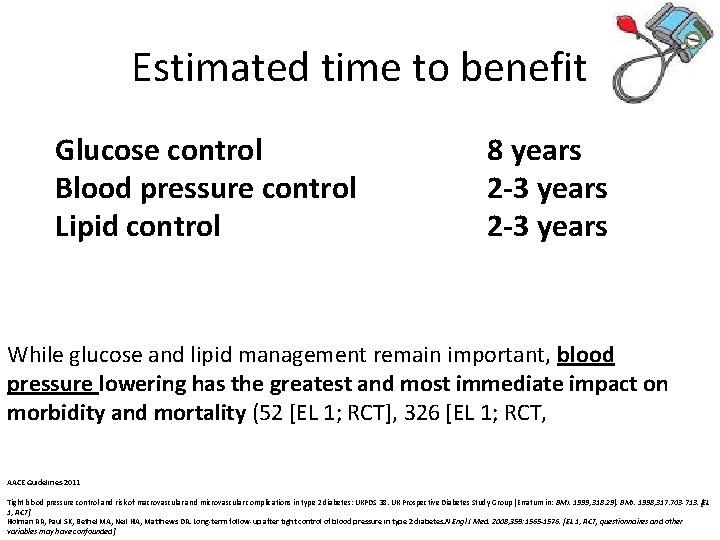
Estimated time to benefit Glucose control Blood pressure control Lipid control 8 years 2 -3 years While glucose and lipid management remain important, blood pressure lowering has the greatest and most immediate impact on morbidity and mortality (52 [EL 1; RCT], 326 [EL 1; RCT, AACE Guidelines-2011 Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group [Erratum in: BMJ. 1999; 318: 29]. BMJ. 1998; 317: 703 -713. [EL 1; RCT] Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008; 359: 1565 -1576. [EL 1; RCT, questionnaires and other variables may have confounded]
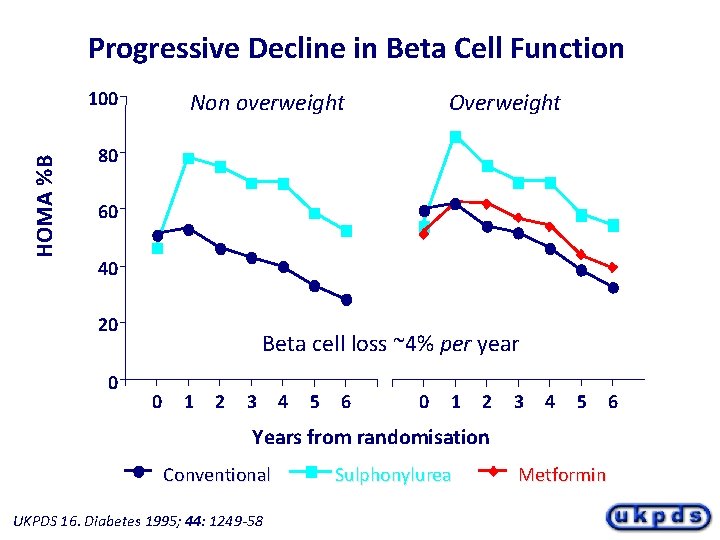
Progressive Decline in Beta Cell Function HOMA %B 100 Non overweight Overweight 80 60 40 20 0 Beta cell loss ~4% per year 0 1 2 3 4 5 6 0 1 2 3 4 5 Years from randomisation Conventional UKPDS 16. Diabetes 1995; 44: 1249 -58 Sulphonylurea Metformin 6
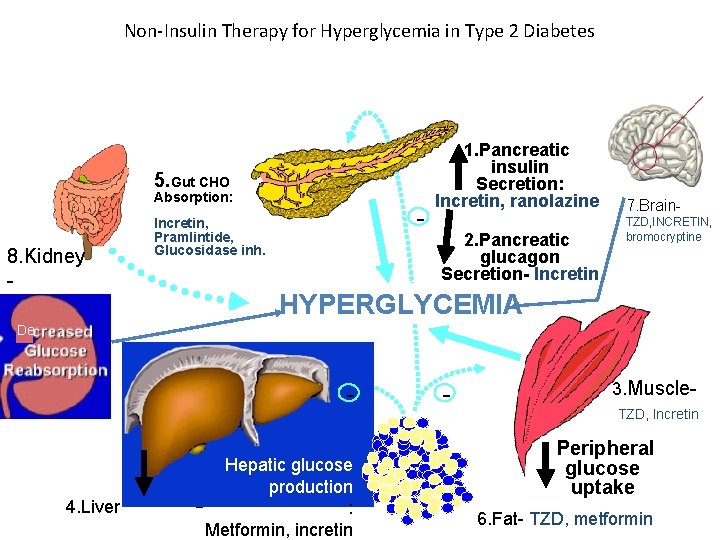
Non-Insulin Therapy for Hyperglycemia in Type 2 Diabetes 5. Gut CHO Absorption: 8. Kidney SGLT 2 - Incretin, Pramlintide, Glucosidase inh. 1. Pancreatic insulin Secretion: Incretin, ranolazine 2. Pancreatic glucagon Secretion- Incretin 7. Brain. TZD, INCRETIN, bromocryptine HYPERGLYCEMIA De - 4. Liver Hepatic glucose production : Metformin, incretin - 3. Muscle. TZD, Incretin Peripheral glucose uptake 6. Fat- TZD, metformin

Metformin-The Pinnacle of Sweet Success
BIGuanides Metformin • • Cardiovascular benefit Decrease A 1 c by 1. 0 – 2. 0 No hypoglycemia Cannot be used in renal failure (Cr Cl < 30 ml/min, s. creat > 1. 5 in males, > 1. 4 in females) • Adverse effects: nausea, vomiting, diarrhea, lactic acidosis, B 12 deficiency • Generic available • Use Metformin SA!!!!

https: //www. aace. com/files/algorithm-07 -11 -2013. pdf p. 12 accessed 12. 16. 13
AACE Metformin Recommendations This limitation has been challenged, however, and lower doses have been proposed for patients with moderate renal insufficiency (126). The AACE agrees with the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease recommendations, which state that metformin should be continued in patients Ø with an e. GFR ≥ 45 m. L/min/1. 73 m 2 (GFR categories G 1 -G 3 a), Ø that its use should be reviewed in those with an e. GFR of 30 to 44 m. L/min/1. 73 m 2 (GFR category G 3 b), Ø and that it should be discontinued in patients with an e. GFR <30 m. L/min/1. 73 m 2 (GFR categories G 4 -G 5) (127).

METFORMIN-Lactic acidosis • Conditions that cause a hypoxemic state: – Renal insufficiency – Concurrent liver disease or alcohol abuse (sgot/sgpt 2 x ULN) – Heart failure (stage III & IV) – History of lactic acidosis – Decreased tissue perfusion or hemodynamic instability – Hypoxic or acute illness

Sulfonylureas • Increase insulin secretion • Decrease A 1 c by 1. 0 – 2. 0 • Risk of hypoglycemia(5 x that of metformin only) • Can be used in mild renal failure, however risk of hypoglycemia more • Skip a meal, skip a dose • Adverse effects: minimal • Generics available
Sulfonylureas • Glipizide – Short acting-need to take ½ hr. prior to meal – Lesser risk of hypoglycemia – Safer than other sulfonylureas in older subjects – Can be used in mild renal failure(Cr. Cl <10) – Change to other medications if renal failure worsens or if hypoglycemia with renal failure

Sulfonylureas • Glyburide – – Longer acting-can take right w/meal Stronger potency than glipizide Increase risk of hypoglycemia Not recommended if Cr Cl < 50 ml/min • Glimepiride (generic now) – Can given once daily dose with meal – Can be used in mild renal failure(Cr. Cl <10)

Use of sulfonylurea as second-line therapy for type 2 diabetes generated glycemic control and QALYs comparable with those associated with other agents but at lower cost. Diabetes Care February 26, 2014 http: //care. diabetesjournals. org/content/early/2014/02/18/dc 13 -1901. long
Nonsulfonylurea Secretagogue • • • Repaglinide (Prandin), Nateglinide (Starlix) MOA o Stimulate glucose-dependent release of insulin by closing the ATP-dependent K+ channels of the pancreatic beta-cells o Shorter half life than SU A 1 c lowering o 0. 5 -1% If the meal is skipped, then omit dose Take medication just prior to eating a carbohydrate meal. Cont’d…

Nonsulfonylurea Secretagogue …cont’d • Indications o Monotherapy with diet o Combination with metformin & TZD • Benefits o o Decrease in post-prandial glucose Short half-life May demonstrate less hypoglycemia than SU Use Caution: co-administration of repaglinide with gemfibrozil is contraindicated. (Gemfibrozil is a hepatic enzyme inducer, therefore repaglinide level could be increased). Cont’d…

Nonsulfonylurea Secretagogue …cont’d • Patient Type o Pt with more predominant post-prandial hyperglycemia o Pt with hypoglycemia episodes on SU o Pt with less consistent food plan o Not for the pt with SU failure & persistently above glycemic goals

Thiazolidinediones (TZDs) Pioglitazone (Actos) & Rosiglitazone (Avandia) o o o Alters transcription of genes that regulate carb and lipid metabolism Increase insulin stimulated glucose uptake by muscle cells Decrease insulin resistance in peripheral tissues Cont’d…
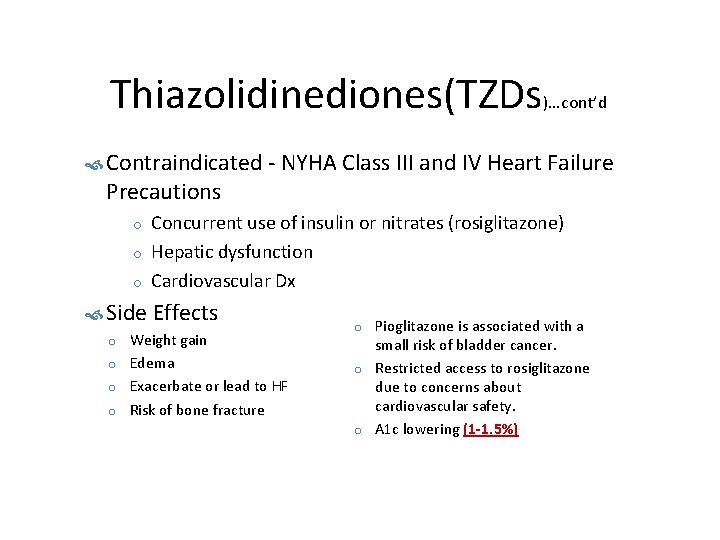
Thiazolidinediones(TZDs)…cont’d Contraindicated - NYHA Class III and IV Heart Failure Precautions o o o Concurrent use of insulin or nitrates (rosiglitazone) Hepatic dysfunction Cardiovascular Dx Side Effects o o Weight gain Edema Exacerbate or lead to HF Risk of bone fracture o o o Pioglitazone is associated with a small risk of bladder cancer. Restricted access to rosiglitazone due to concerns about cardiovascular safety. A 1 c lowering (1 -1. 5%)
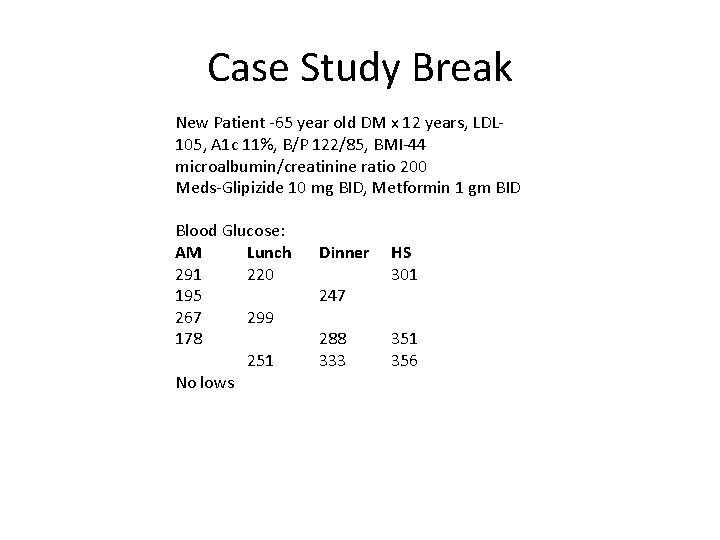
Case Study Break New Patient -65 year old DM x 12 years, LDL 105, A 1 c 11%, B/P 122/85, BMI-44 microalbumin/creatinine ratio 200 Meds-Glipizide 10 mg BID, Metformin 1 gm BID Blood Glucose: AM Lunch 291 220 195 267 299 178 251 No lows Dinner 247 288 333 HS 301 356
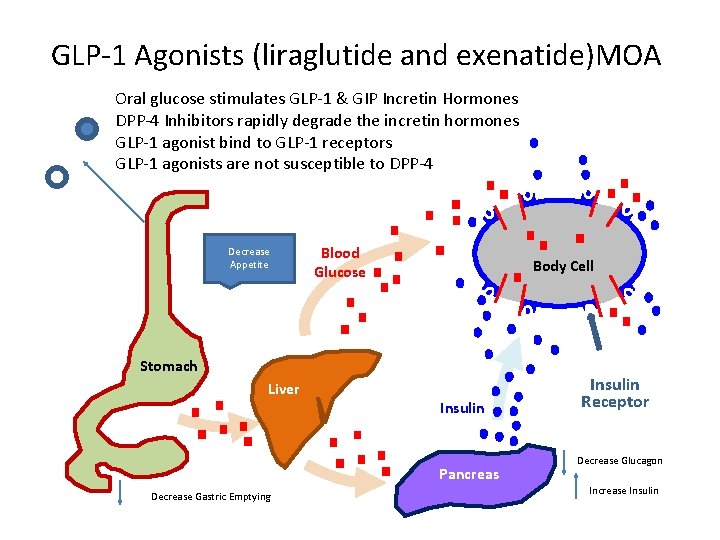
GLP-1 Agonists (liraglutide and exenatide)MOA Oral glucose stimulates GLP-1 & GIP Incretin Hormones DPP-4 Inhibitors rapidly degrade the incretin hormones GLP-1 agonist bind to GLP-1 receptors GLP-1 agonists are not susceptible to DPP-4 Decrease Appetite Blood Glucose Body Cell Stomach Liver Insulin Pancreas Decrease Gastric Emptying Insulin Receptor Decrease Glucagon Increase Insulin

GLP-1 Agonists • A 1 c lowering- Adding a GLP-1 agonist to metformin or SU resulted in mean decrease in A 1 c ranging from 0. 8 -1. 0% o Not susceptible to DPP-4 degradation o Increases Insulin secretion only in response to glucose load or elevated glucose concentration o Given as a subcutaneous injection • Side effects: nausea/hypoglycemia/wt. loss o Avoid use in patients with history of pancreatitis o Liraglutide is associated with thyroid C cell tumors in rodents, but unknown in humans VA/Do. D Guidelines http: //www. healthquality. va. gov/diabetes/DM 2010_FUL-v 4 e. pdf
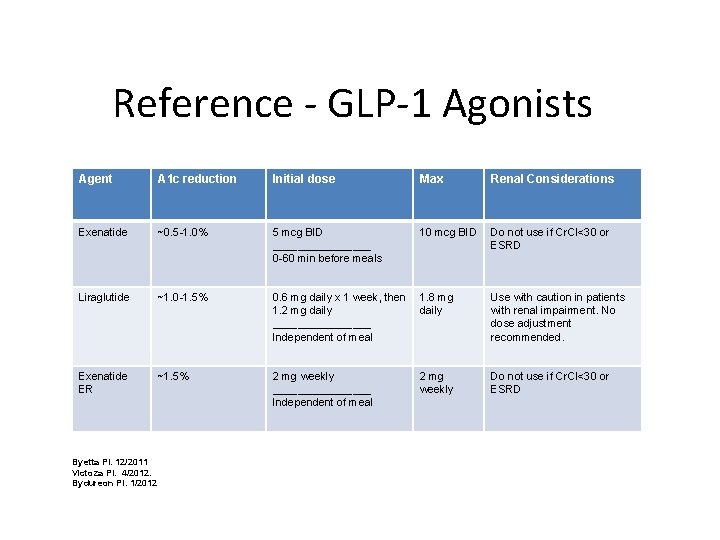
Reference - GLP-1 Agonists Agent A 1 c reduction Initial dose Max Renal Considerations Exenatide ~0. 5 -1. 0% 5 mcg BID ________ 0 -60 min before meals 10 mcg BID Do not use if Cr. Cl<30 or ESRD Liraglutide ~1. 0 -1. 5% 0. 6 mg daily x 1 week, then 1. 2 mg daily ________ Independent of meal 1. 8 mg daily Use with caution in patients with renal impairment. No dose adjustment recommended. Exenatide ER ~1. 5% 2 mg weekly ________ Independent of meal 2 mg weekly Do not use if Cr. Cl<30 or ESRD Byetta PI. 12/2011 Victoza PI. 4/2012. Bydureon PI. 1/2012
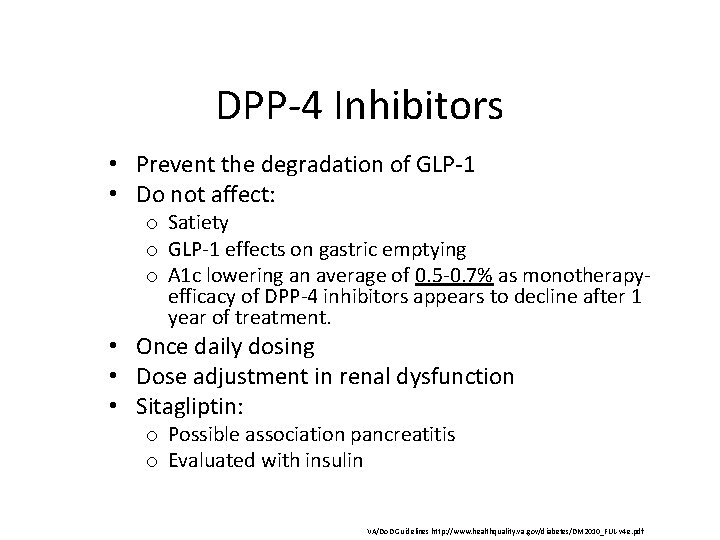
DPP-4 Inhibitors • Prevent the degradation of GLP-1 • Do not affect: o Satiety o GLP-1 effects on gastric emptying o A 1 c lowering an average of 0. 5 -0. 7% as monotherapyefficacy of DPP-4 inhibitors appears to decline after 1 year of treatment. • Once daily dosing • Dose adjustment in renal dysfunction • Sitagliptin: o Possible association pancreatitis o Evaluated with insulin VA/Do. D Guidelines http: //www. healthquality. va. gov/diabetes/DM 2010_FUL-v 4 e. pdf
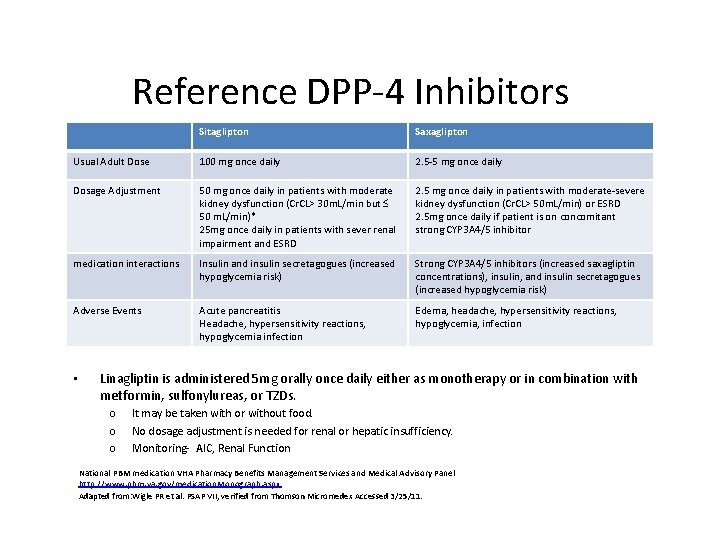
Reference DPP-4 Inhibitors Sitaglipton Saxaglipton Usual Adult Dose 100 mg once daily 2. 5 -5 mg once daily Dosage Adjustment 50 mg once daily in patients with moderate kidney dysfunction (Cr. CL> 30 m. L/min but £ 50 m. L/min)* 25 mg once daily in patients with sever renal impairment and ESRD 2. 5 mg once daily in patients with moderate-severe kidney dysfunction (Cr. CL> 50 m. L/min) or ESRD 2. 5 mg once daily if patient is on concomitant strong CYP 3 A 4/5 inhibitor medication interactions Insulin and insulin secretagogues (increased hypoglycemia risk) Strong CYP 3 A 4/5 inhibitors (increased saxagliptin concentrations), insulin, and insulin secretagogues (increased hypoglycemia risk) Adverse Events Acute pancreatitis Headache, hypersensitivity reactions, hypoglycemia infection Edema, headache, hypersensitivity reactions, hypoglycemia, infection • Linagliptin is administered 5 mg orally once daily either as monotherapy or in combination with metformin, sulfonylureas, or TZDs. o o o It may be taken with or without food. No dosage adjustment is needed for renal or hepatic insufficiency. Monitoring- AIC, Renal Function National PBM medication VHA Pharmacy Benefits Management Services and Medical Advisory Panel http: //www. pbm. va. gov/medication. Monograph. aspx Adapted from: Wigle PR et al. PSAP VII, verified from Thomson Micromedex Accessed 3/25/11.

Discontinuation Criteria • These agents are not to be used in patients with history of pancreatitis. • Pancreatitis has been reported with the DPP-4 inhibitors. Monitor patients carefully for the development of pancreatitis after initiation or dose increases of agent. Discontinue agent if pancreatitis is suspected while using these products. • Serious allergic and hypersensitivity reactions (e. g. anaphylaxis, angioedema, exfoliative skin conditions including Stevens-Johnson syndrome) have been reported with the DPP-4 inhibitors. If these reactions occur, discontinue agent and initiate alternative treatment for diabetes. • Consider lowering insulin or Sulfonylurea dose if DPP 4 inhibitor is initiated. Dipeptidyl-peptidase-4 (DPP-4) Inhibitors: Sitagliptin, Saxagliptin, and Linagliptin Criteria for Use http: //www. pbm. va. gov/Criteria. For. Use. aspx National PBM medication VHA Pharmacy Benefits Management Services and Medical Advisory Panel http: //www. pbm. va. gov/medication. Monograph. aspx
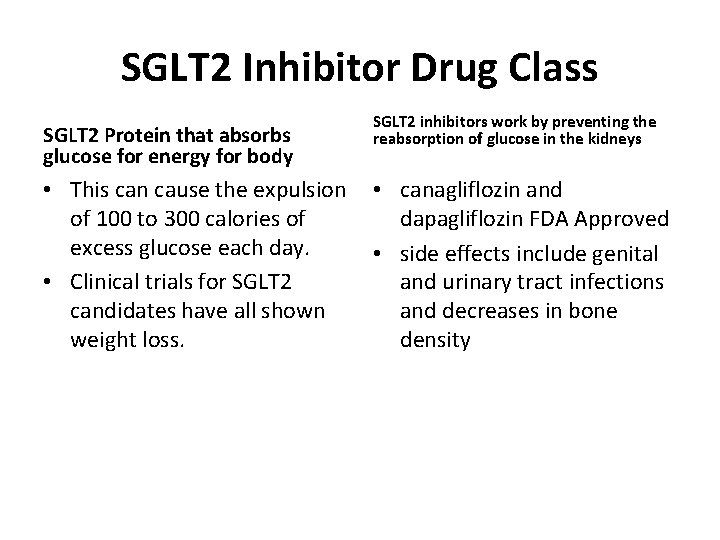
SGLT 2 Inhibitor Drug Class SGLT 2 Protein that absorbs glucose for energy for body SGLT 2 inhibitors work by preventing the reabsorption of glucose in the kidneys • This can cause the expulsion • canagliflozin and of 100 to 300 calories of dapagliflozin FDA Approved excess glucose each day. • side effects include genital and urinary tract infections • Clinical trials for SGLT 2 candidates have all shown and decreases in bone weight loss. density
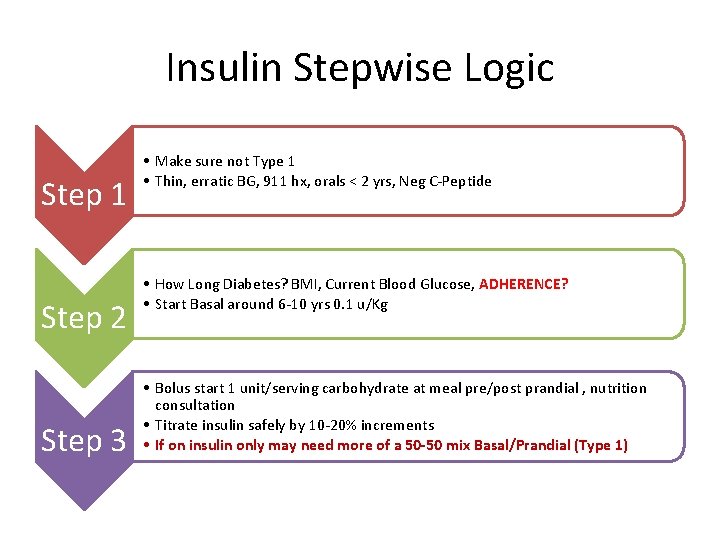
Insulin Stepwise Logic Step 1 Step 2 Step 3 • Make sure not Type 1 • Thin, erratic BG, 911 hx, orals < 2 yrs, Neg C-Peptide • How Long Diabetes? BMI, Current Blood Glucose, ADHERENCE? • Start Basal around 6 -10 yrs 0. 1 u/Kg • Bolus start 1 unit/serving carbohydrate at meal pre/post prandial , nutrition consultation • Titrate insulin safely by 10 -20% increments • If on insulin only may need more of a 50 -50 mix Basal/Prandial (Type 1)
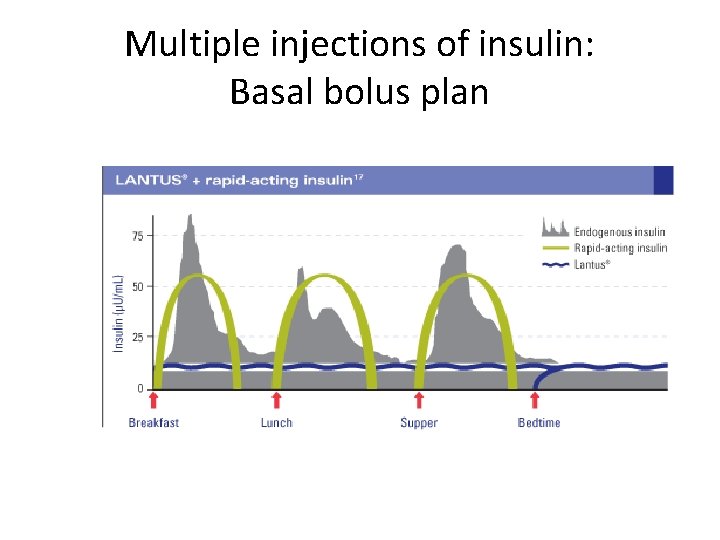
Multiple injections of insulin: Basal bolus plan
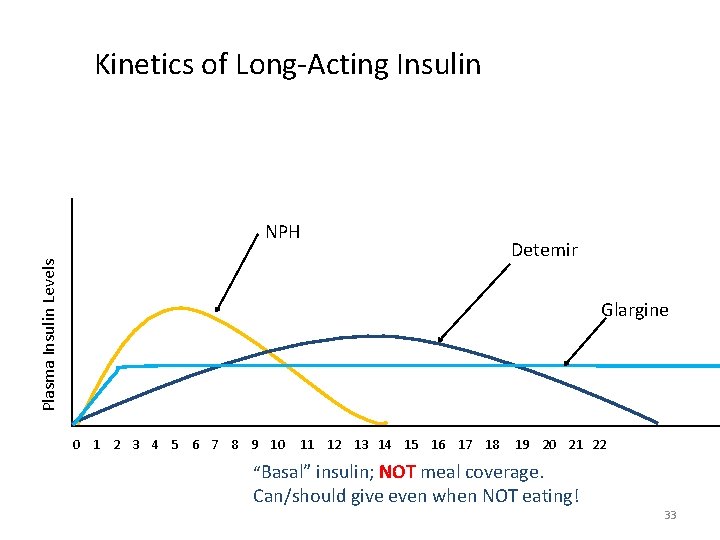
Kinetics of Long-Acting Insulin Plasma Insulin Levels NPH Detemir Glargine 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 “Basal” insulin; NOT meal coverage. Can/should give even when NOT eating! 33

Initiating Basal Insulin • Generally start 0. 1 -0. 2 Units/kg • Titrate 10 -20% at a time (every 2 -7 days) until target range, or any low blood sugar • Rotate injection sites • Do not shake N too hard • Check fasting and before meal Blood Sugars to identify if basal dose correct
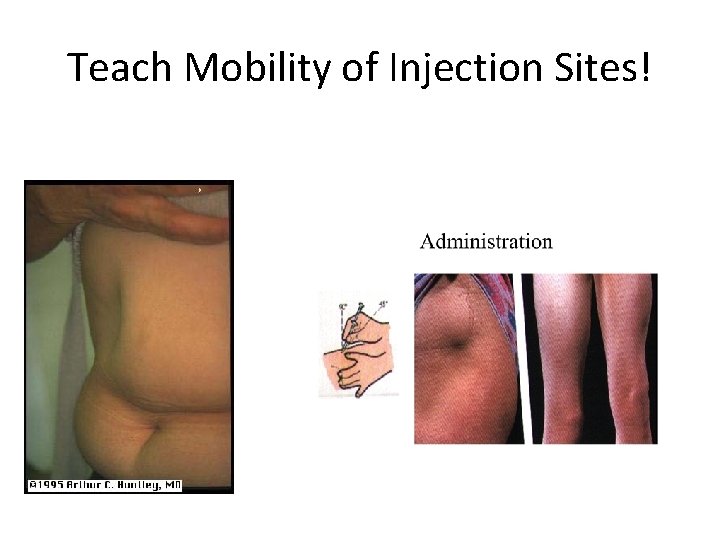
Teach Mobility of Injection Sites!

Prandial Insulin • No set rules for initiation but safe practice is 1 unit per CHO consumed • May be able to start daily or twice daily • Titrate based on 2 hour pre/ post-prandial level
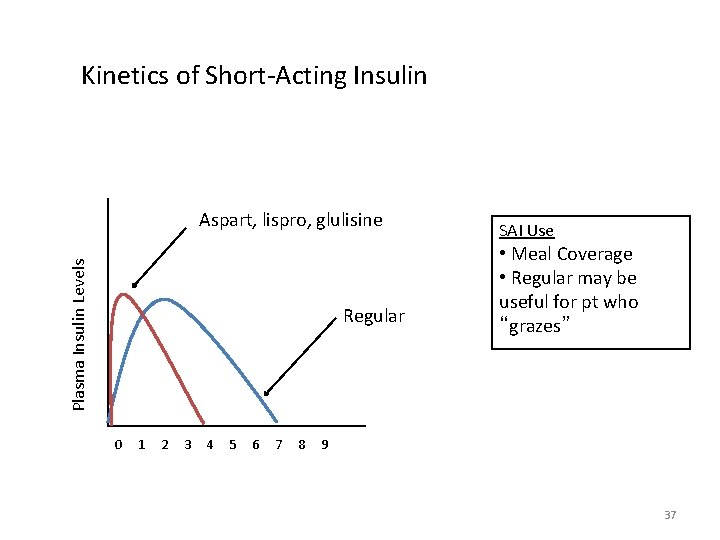
Kinetics of Short-Acting Insulin Plasma Insulin Levels Aspart, lispro, glulisine Regular SAI Use • Meal Coverage • Regular may be useful for pt who “grazes” 0 1 2 3 4 5 6 7 8 9 37
Hypoglycemia • Some dangerous sequelae of hypoglycemia: – tachycardia – bradyarrhythmias – frequent ventricular ectopic beats – ST depression – T-wave flattening – QT prolongation Kodl C. T. & Seaquist E. R. (2008) Practical strategies to normalize hyperglycemia without undue hypoglycemia in Type 2 diabetes mellitus. Current Diabetes Reports 8, 375 - 382.
Hypoglycemia (cont. ) • Studies have also shown the effects of diminished subsequent hypoglycemia response after a first episode (even in those without diabetes). • The compensatory increase in cortisol production during a first hypoglycemic episode may play a central role in minimizing the protective hormonal responses during a subsequent episode Davis S. N. Mann S. Briscoe V. J. Ertl A. C. & Tate D. B. (2009) The effects of intensive therapy and antecedent hypoglycemia on counter regulatory responses to hypoglycemia in type 2 diabetes. Diabetes 58 701 -705.
Hypoglycemia (cont. ) • If the blood glucose falls to 50 mg/d. L (2. 8 mmol/L), transient cognitive deficits may also ensue, which can result in falls or aspiration. • If the blood glucose falls <40 mg/d. L (2. 2 mmol/L), seizure or coma may ensue. Ben-Ami, H. , Nagachandran, P. , Mendelson, A. , and Edoute, Y. 1999. Drug-induced hypoglycemic coma in 102 diabetic patients. Arch. Intern. Med. 159: 281– 284. Cryer, P. E. 2001. The prevention and correction of hypoglycemia. In Handbook of physiology. Section 7, The endocrine system. Volume 2, The endocrine pancreas and regulation of metabolism. L. S. Jefferson and A. D. Cherrington, editors. Oxford University Press. New York, USA. 1057– 1092.
Key Take Home Points • View BS’s in a daily pattern • Use ½ dose of SU-Clinically effective dose • Use Metformin or Metformin SA-think compelling reason NOT to be on it • Start basal insulin early (UKPDS-6 yrs-50%) 0. 1 U/kg • 9 -9 -9 Rule of starting basal insulin • Start bolus insulin early if needed 12~15 yrs-1 unit per CHOcheck 2 hr PP levels • Titrate insulin 10~20% at a time • If not on orals 50 -50% mix basal/bolus to control blood glucose • Avoid Hypoglycemia

Resources
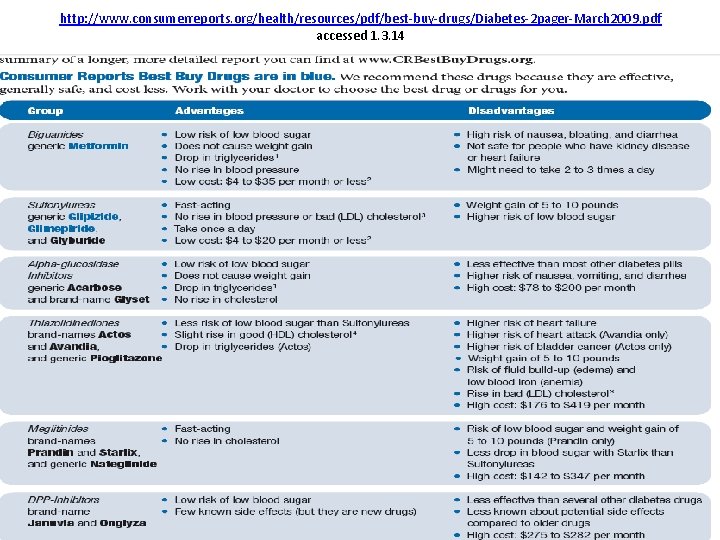
http: //www. consumerreports. org/health/resources/pdf/best-buy-drugs/Diabetes-2 pager-March 2009. pdf accessed 1. 3. 14

Types of Insulin Onset Peak Duration Comment 10 -20 min 1 -3 hours 3 -5 hrs Both types are used in insulin pumps, inject 10 min prior to meals 15 -30 min to 2 ½ hours 3 -5 hrs 30 min-1 hr 2 -5 hours 5 -8 hrs Increased risk of nocturnal hypoglycemia compared to Novolog 1 -2 hrs 4 -12 hours 18 -24 hrs Usually given twice daily; Increased risk of nocturnal hypoglycemia compared to Novolog 1 -1 ½ hrs None 20 -24 hrs Lantus-usually given once a day Levemir-Once or twice a day 10 -20 min 1 -4 hrs Up to 24 hrs May be given up to three times per day. Rapid Acting Novolog Humalog Covers insulin needs for meals eaten within 10 -30 mins, or as advised by physician Short Acting Regular (R) Intermediate Acting NPH (N) Long Acting Lantus- pen or vial Levemir-pen or vial Pre-mixed Novolog Mix 70/30 http: //diabeteshealth. com/media/pdfs/PRG 0113/Insulin. pdf
- Slides: 44