BENZENE Primary analysis revealed benzene had an a
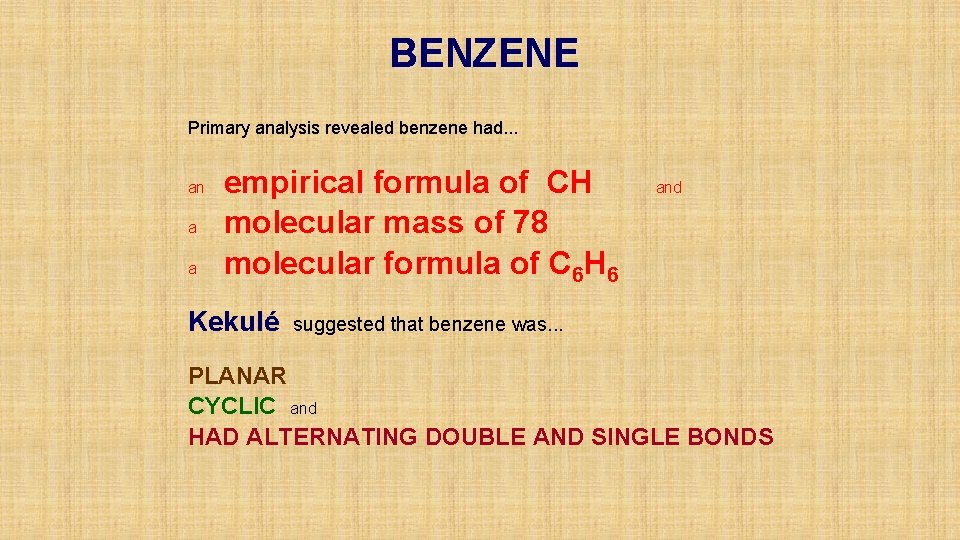
BENZENE Primary analysis revealed benzene had. . . an a a empirical formula of CH molecular mass of 78 molecular formula of C 6 H 6 Kekulé and suggested that benzene was. . . PLANAR CYCLIC and HAD ALTERNATING DOUBLE AND SINGLE BONDS

STRUCTURE OF BENZENE HOWEVER. . . • it did not readily undergo electrophilic addition - no true C=C bond • only one 1, 2 disubstituted product existed • all six C—C bond lengths were similar; C=C bonds are shorter than C-C • the ring was thermodynamically more stable than expected
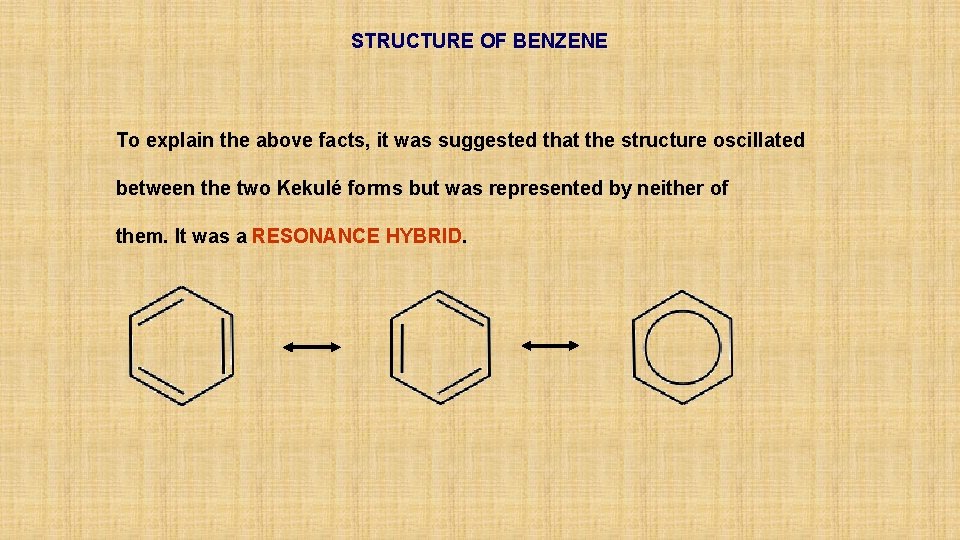
STRUCTURE OF BENZENE To explain the above facts, it was suggested that the structure oscillated between the two Kekulé forms but was represented by neither of them. It was a RESONANCE HYBRID.

THERMODYNAMIC EVIDENCE FOR STABILITY When unsaturated hydrocarbons are reduced to the corresponding saturated compound, energy is released. The amount of heat liberated per mole (enthalpy of hydrogenation) can be measured.
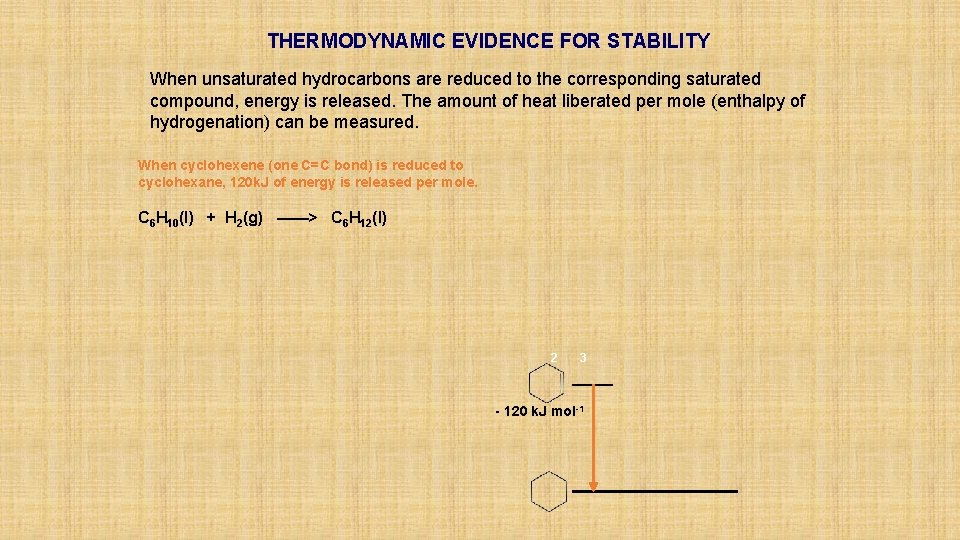
THERMODYNAMIC EVIDENCE FOR STABILITY When unsaturated hydrocarbons are reduced to the corresponding saturated compound, energy is released. The amount of heat liberated per mole (enthalpy of hydrogenation) can be measured. When cyclohexene (one C=C bond) is reduced to cyclohexane, 120 k. J of energy is released per mole. C 6 H 10(l) + H 2(g) ——> C 6 H 12(l) 2 3 - 120 k. J mol-1
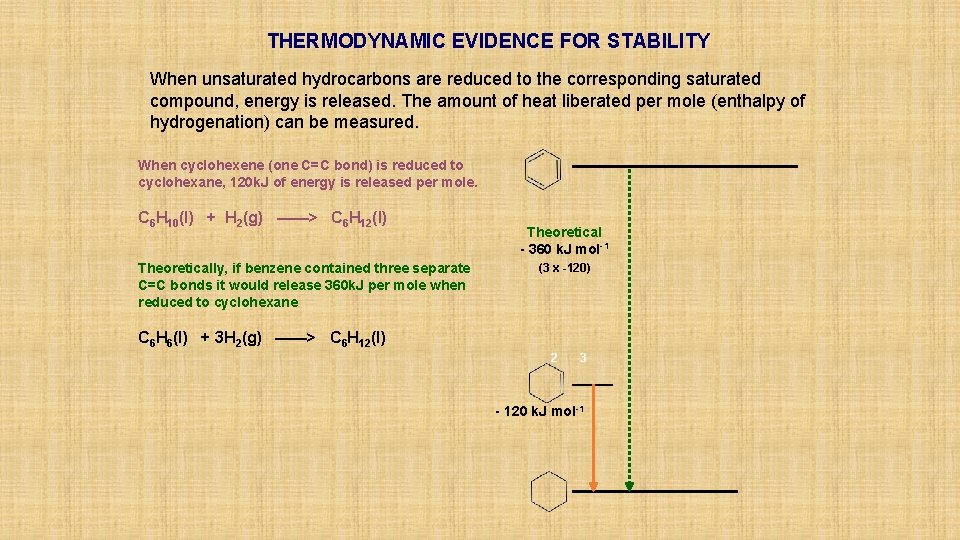
THERMODYNAMIC EVIDENCE FOR STABILITY When unsaturated hydrocarbons are reduced to the corresponding saturated compound, energy is released. The amount of heat liberated per mole (enthalpy of hydrogenation) can be measured. When cyclohexene (one C=C bond) is reduced to cyclohexane, 120 k. J of energy is released per mole. C 6 H 10(l) + H 2(g) ——> C 6 H 12(l) Theoretically, if benzene contained three separate C=C bonds it would release 360 k. J per mole when reduced to cyclohexane Theoretical - 360 k. J mol-1 (3 x -120) C 6 H 6(l) + 3 H 2(g) ——> C 6 H 12(l) 2 3 - 120 k. J mol-1
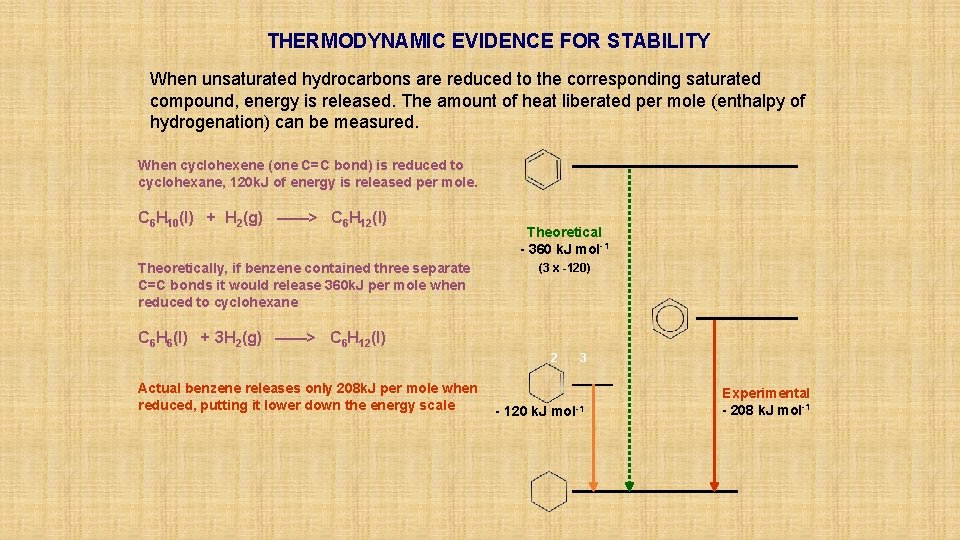
THERMODYNAMIC EVIDENCE FOR STABILITY When unsaturated hydrocarbons are reduced to the corresponding saturated compound, energy is released. The amount of heat liberated per mole (enthalpy of hydrogenation) can be measured. When cyclohexene (one C=C bond) is reduced to cyclohexane, 120 k. J of energy is released per mole. C 6 H 10(l) + H 2(g) ——> C 6 H 12(l) Theoretically, if benzene contained three separate C=C bonds it would release 360 k. J per mole when reduced to cyclohexane Theoretical - 360 k. J mol-1 (3 x -120) C 6 H 6(l) + 3 H 2(g) ——> C 6 H 12(l) 2 Actual benzene releases only 208 k. J per mole when reduced, putting it lower down the energy scale 3 - 120 k. J mol-1 Experimental - 208 k. J mol-1
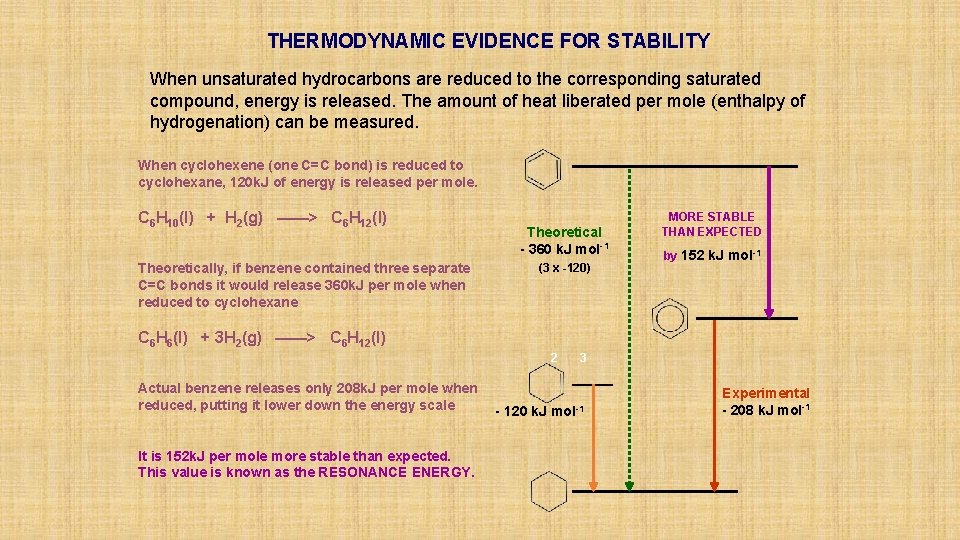
THERMODYNAMIC EVIDENCE FOR STABILITY When unsaturated hydrocarbons are reduced to the corresponding saturated compound, energy is released. The amount of heat liberated per mole (enthalpy of hydrogenation) can be measured. When cyclohexene (one C=C bond) is reduced to cyclohexane, 120 k. J of energy is released per mole. C 6 H 10(l) + H 2(g) ——> C 6 H 12(l) Theoretically, if benzene contained three separate C=C bonds it would release 360 k. J per mole when reduced to cyclohexane Theoretical - 360 k. J mol-1 (3 x -120) MORE STABLE THAN EXPECTED by 152 k. J mol-1 C 6 H 6(l) + 3 H 2(g) ——> C 6 H 12(l) 2 Actual benzene releases only 208 k. J per mole when reduced, putting it lower down the energy scale It is 152 k. J per mole more stable than expected. This value is known as the RESONANCE ENERGY. 3 - 120 k. J mol-1 Experimental - 208 k. J mol-1
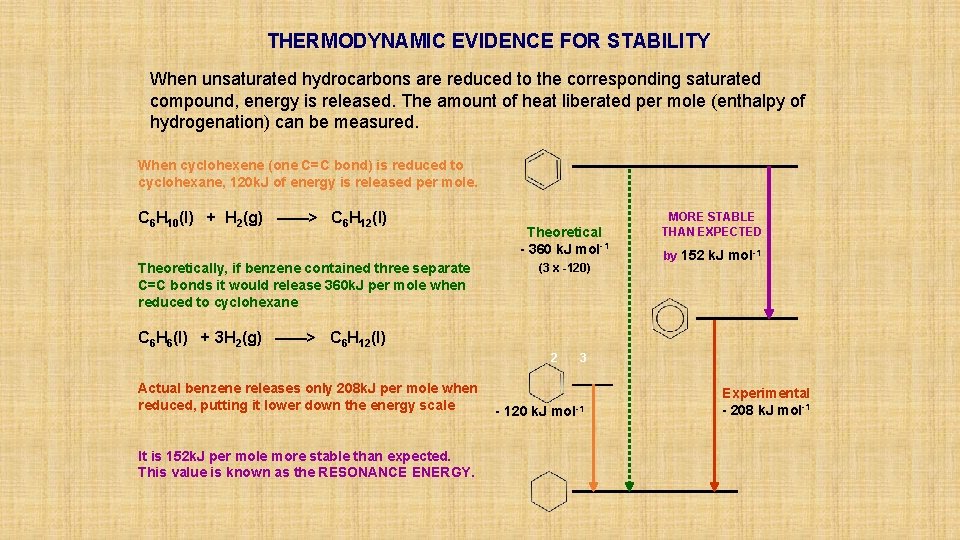
THERMODYNAMIC EVIDENCE FOR STABILITY When unsaturated hydrocarbons are reduced to the corresponding saturated compound, energy is released. The amount of heat liberated per mole (enthalpy of hydrogenation) can be measured. When cyclohexene (one C=C bond) is reduced to cyclohexane, 120 k. J of energy is released per mole. C 6 H 10(l) + H 2(g) ——> C 6 H 12(l) Theoretically, if benzene contained three separate C=C bonds it would release 360 k. J per mole when reduced to cyclohexane Theoretical - 360 k. J mol-1 (3 x -120) MORE STABLE THAN EXPECTED by 152 k. J mol-1 C 6 H 6(l) + 3 H 2(g) ——> C 6 H 12(l) 2 Actual benzene releases only 208 k. J per mole when reduced, putting it lower down the energy scale It is 152 k. J per mole more stable than expected. This value is known as the RESONANCE ENERGY. 3 - 120 k. J mol-1 Experimental - 208 k. J mol-1
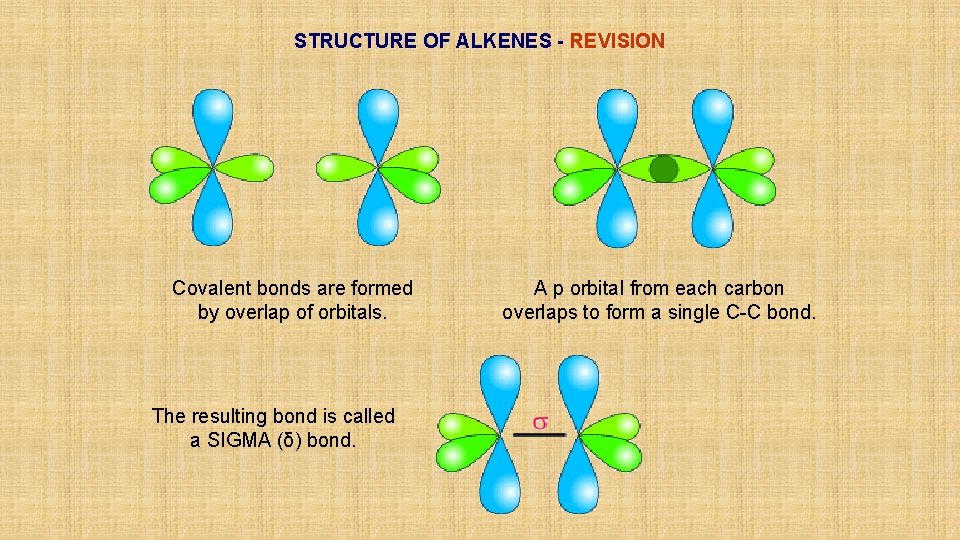
STRUCTURE OF ALKENES - REVISION Covalent bonds are formed by overlap of orbitals. The resulting bond is called a SIGMA (δ) bond. A p orbital from each carbon overlaps to form a single C-C bond.
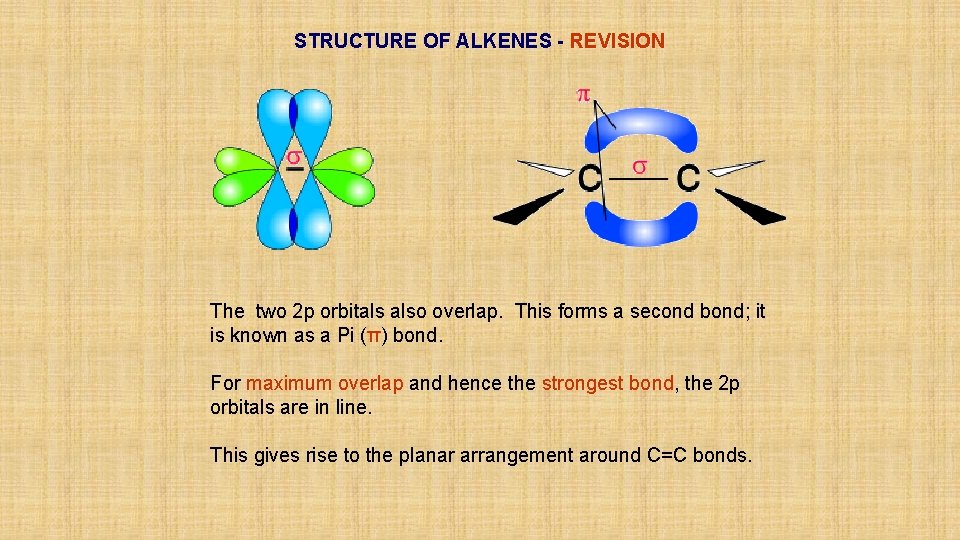
STRUCTURE OF ALKENES - REVISION The two 2 p orbitals also overlap. This forms a second bond; it is known as a Pi (π) bond. For maximum overlap and hence the strongest bond, the 2 p orbitals are in line. This gives rise to the planar arrangement around C=C bonds.
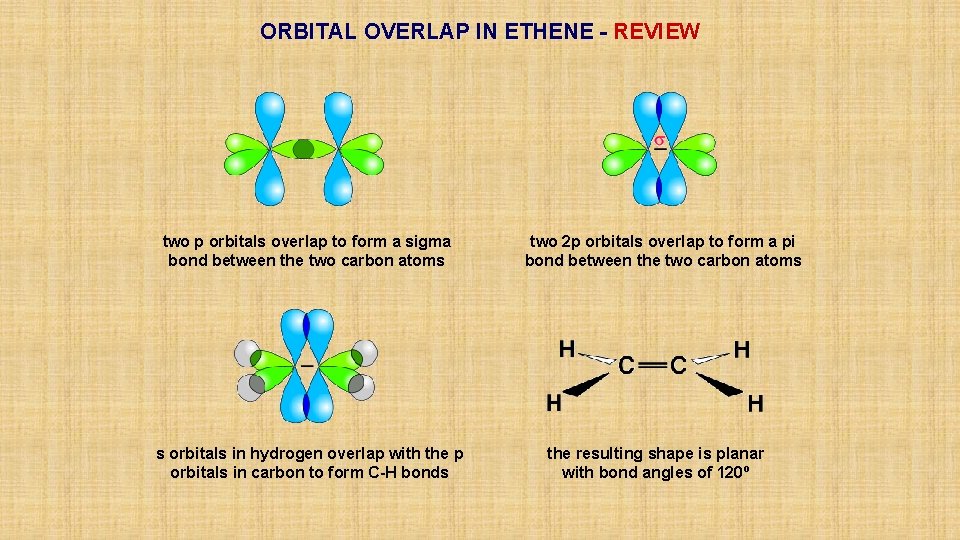
ORBITAL OVERLAP IN ETHENE - REVIEW two p orbitals overlap to form a sigma bond between the two carbon atoms s orbitals in hydrogen overlap with the p orbitals in carbon to form C-H bonds two 2 p orbitals overlap to form a pi bond between the two carbon atoms the resulting shape is planar with bond angles of 120º
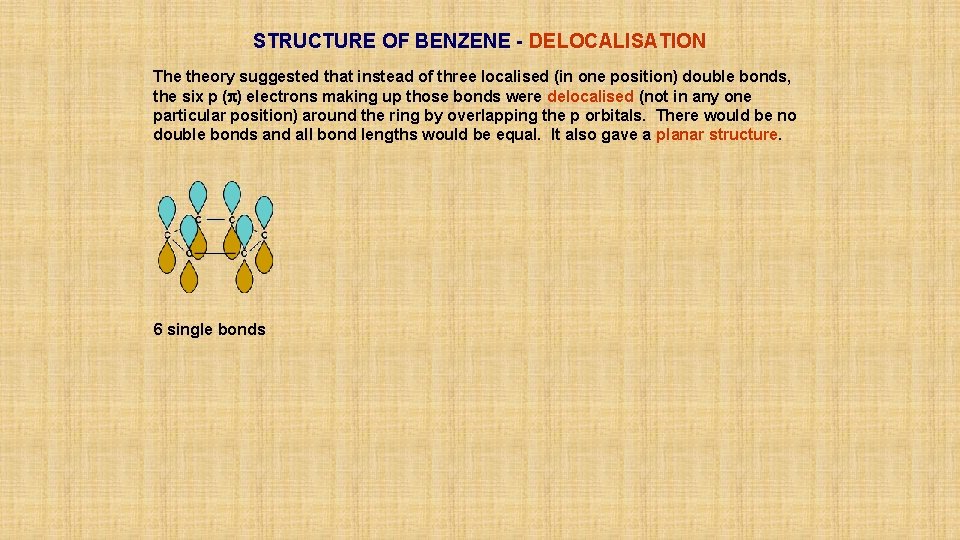
STRUCTURE OF BENZENE - DELOCALISATION The theory suggested that instead of three localised (in one position) double bonds, the six p (p) electrons making up those bonds were delocalised (not in any one particular position) around the ring by overlapping the p orbitals. There would be no double bonds and all bond lengths would be equal. It also gave a planar structure. 6 single bonds
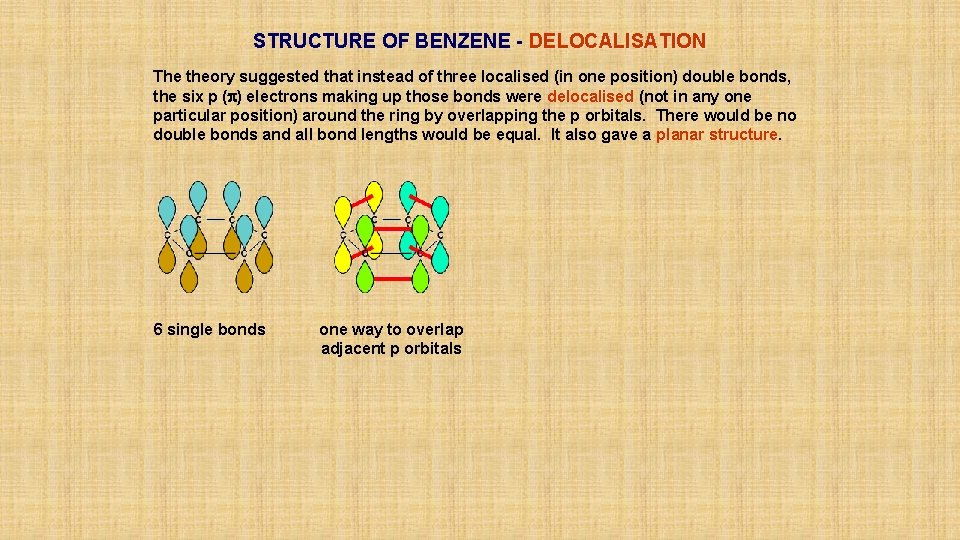
STRUCTURE OF BENZENE - DELOCALISATION The theory suggested that instead of three localised (in one position) double bonds, the six p (p) electrons making up those bonds were delocalised (not in any one particular position) around the ring by overlapping the p orbitals. There would be no double bonds and all bond lengths would be equal. It also gave a planar structure. 6 single bonds one way to overlap adjacent p orbitals
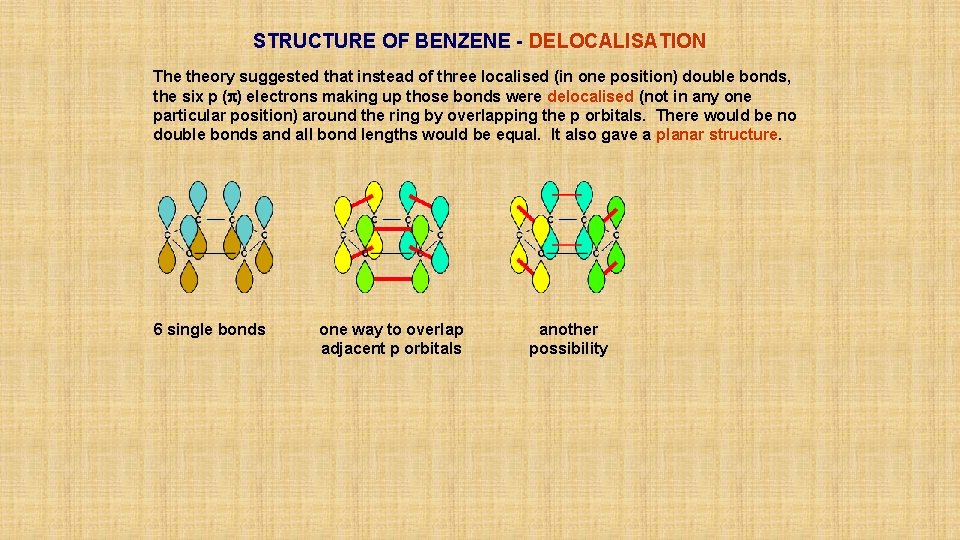
STRUCTURE OF BENZENE - DELOCALISATION The theory suggested that instead of three localised (in one position) double bonds, the six p (p) electrons making up those bonds were delocalised (not in any one particular position) around the ring by overlapping the p orbitals. There would be no double bonds and all bond lengths would be equal. It also gave a planar structure. 6 single bonds one way to overlap adjacent p orbitals another possibility
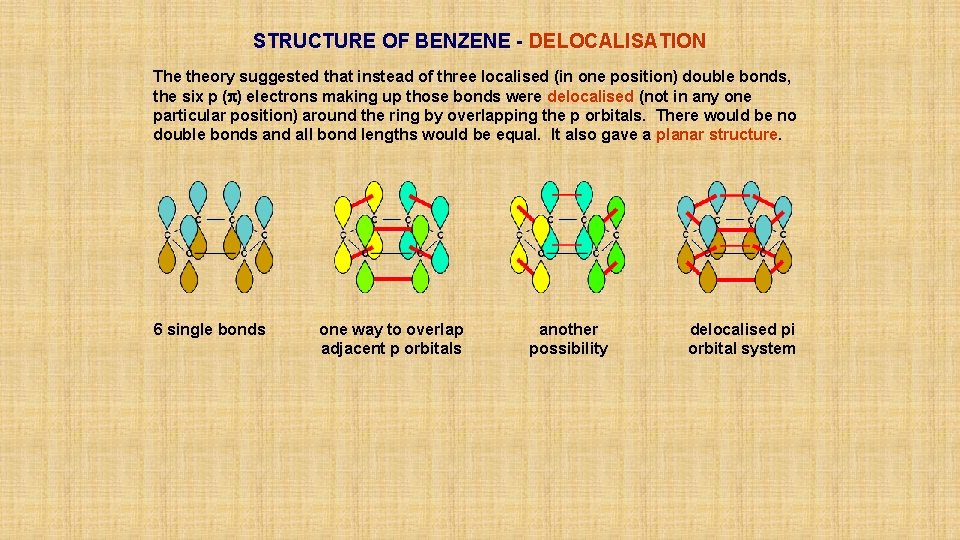
STRUCTURE OF BENZENE - DELOCALISATION The theory suggested that instead of three localised (in one position) double bonds, the six p (p) electrons making up those bonds were delocalised (not in any one particular position) around the ring by overlapping the p orbitals. There would be no double bonds and all bond lengths would be equal. It also gave a planar structure. 6 single bonds one way to overlap adjacent p orbitals another possibility delocalised pi orbital system
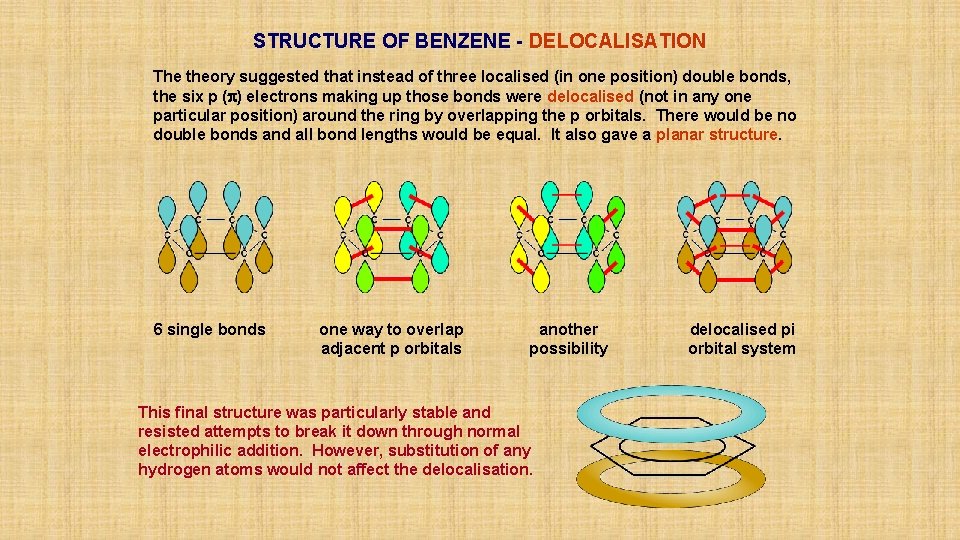
STRUCTURE OF BENZENE - DELOCALISATION The theory suggested that instead of three localised (in one position) double bonds, the six p (p) electrons making up those bonds were delocalised (not in any one particular position) around the ring by overlapping the p orbitals. There would be no double bonds and all bond lengths would be equal. It also gave a planar structure. 6 single bonds one way to overlap adjacent p orbitals another possibility This final structure was particularly stable and resisted attempts to break it down through normal electrophilic addition. However, substitution of any hydrogen atoms would not affect the delocalisation. delocalised pi orbital system
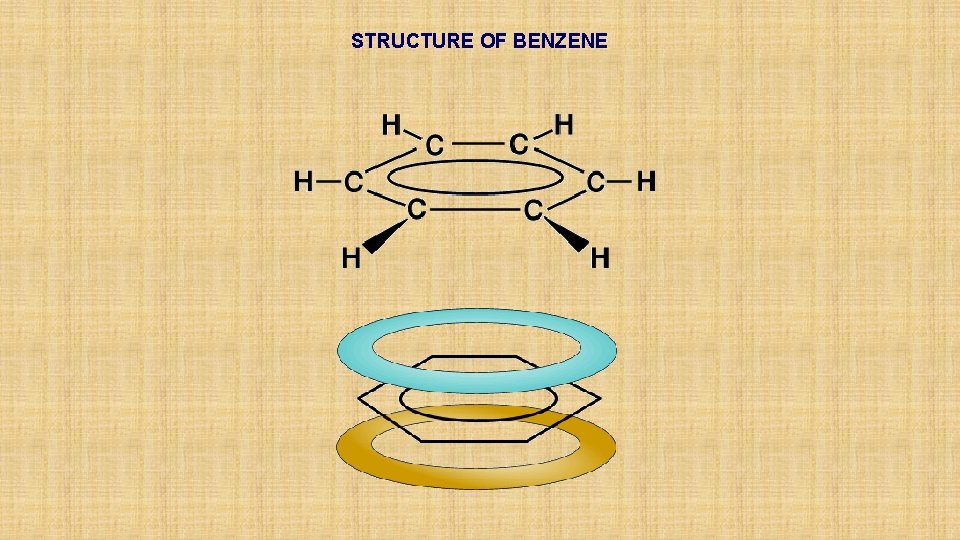
STRUCTURE OF BENZENE

Benzene Molecule • Six carbon atoms joined to form a hexagonal planar ring. • Each carbon has four valence electrons • One of these is used to form a bond with a hydrogen atom. • Two other electrons are used to form sigma bonds with the carbon atoms on the either side.
Benzene Molecule • X-ray analysis showed that all carbon-carbon bonds in benzene are of equal length intermediate between a single and a double bond. • C – C = 0. 154 nm C = 0. 134 nm C – C in benzene = 0. 139 nm
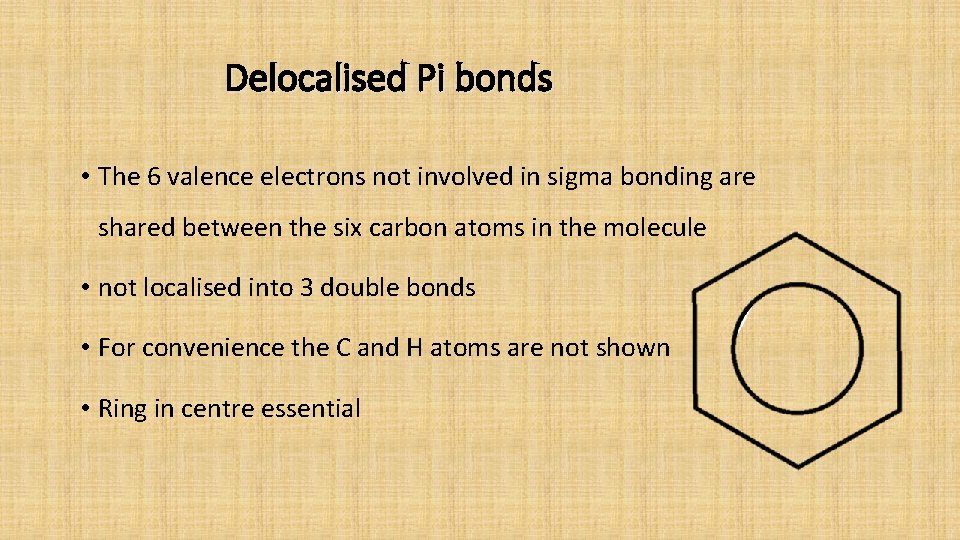
Delocalised Pi bonds • The 6 valence electrons not involved in sigma bonding are shared between the six carbon atoms in the molecule • not localised into 3 double bonds • For convenience the C and H atoms are not shown • Ring in centre essential

Aromatic Compounds • Aromatic compounds are compounds which contain a benzene ring in their molecules. • Benzene C 6 H 6 • Methylbenzene C 7 H 8 • Ethylbenzene C 8 H 10
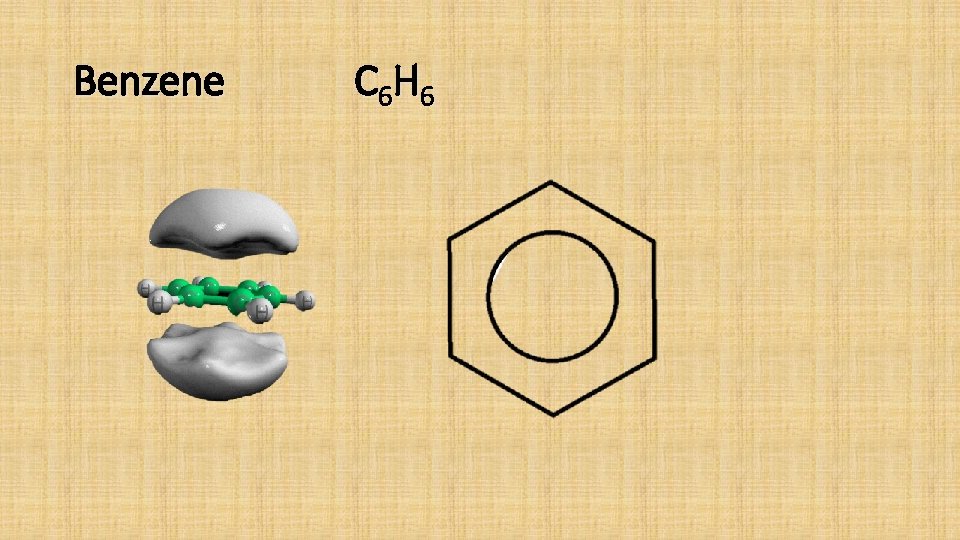
Benzene C 6 H 6
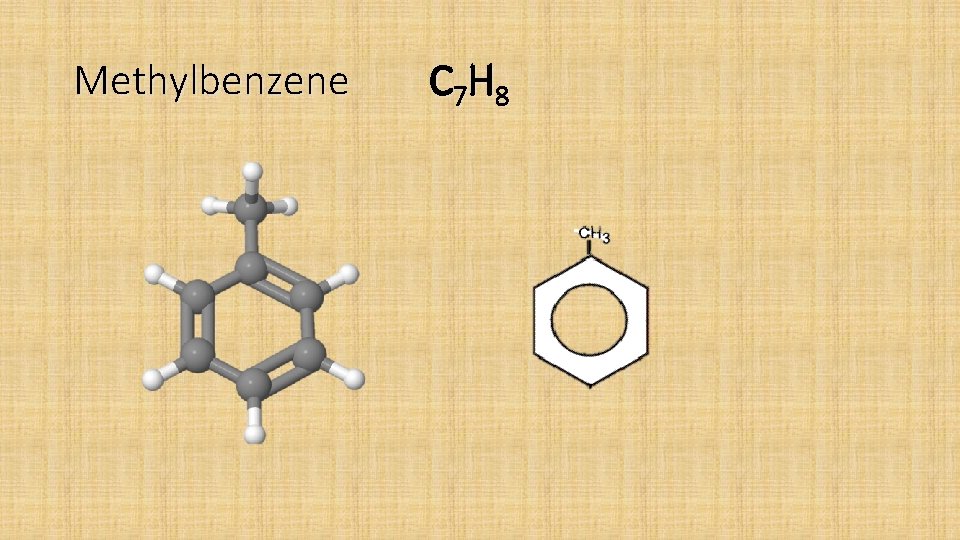
Methylbenzene C 7 H 8
Ethylbenzene C 8 H 10

Physical properties • Physical state: Benzene. methylbenzene and ethylbenzene are liquids • Insoluble in water • Soluble in non-polar solvents such as cyclohexane
- Slides: 26