Benefit Risk Assessment Overview Examples National experience Project

Benefit Risk Assessment • Overview • Examples • National experience Project 3 S Advance workshop for strengthening Pharmacovigilance (PV) Systems and PV preparedness Geneva, 03 -07 December 2018 Viola Macolic Sarinic, WHO HQ, SAV
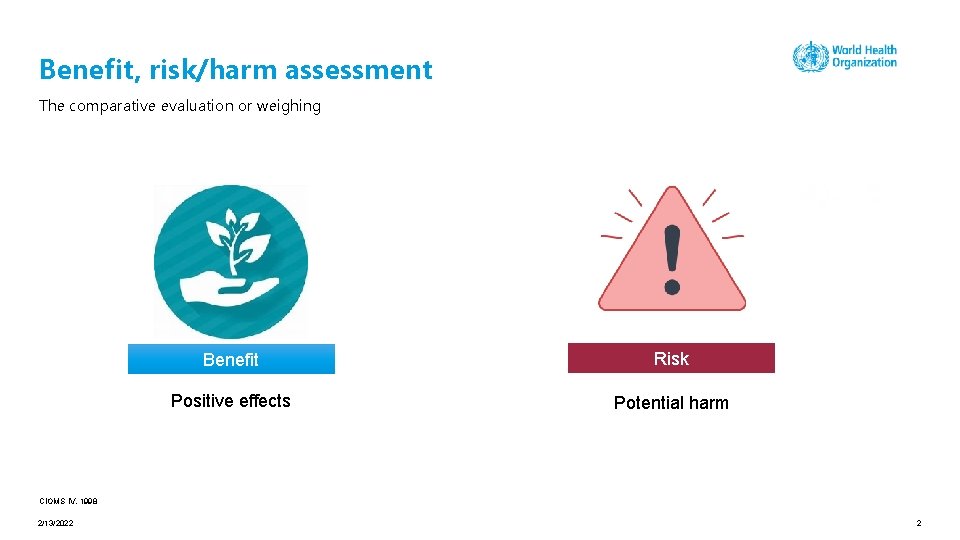
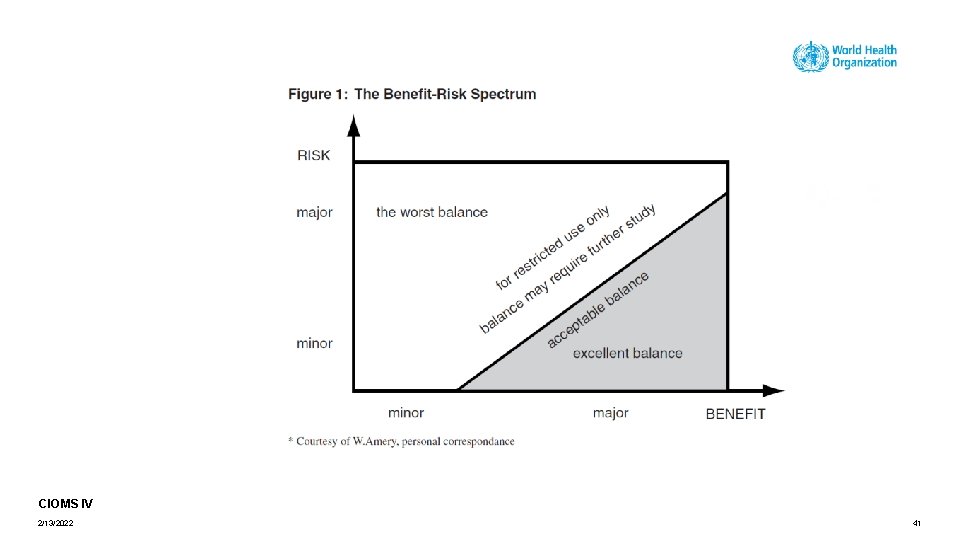
Benefit, risk/harm assessment The comparative evaluation or weighing Benefit Risk Positive effects Potential harm CIOMS IV, 1998 2/13/2022 2

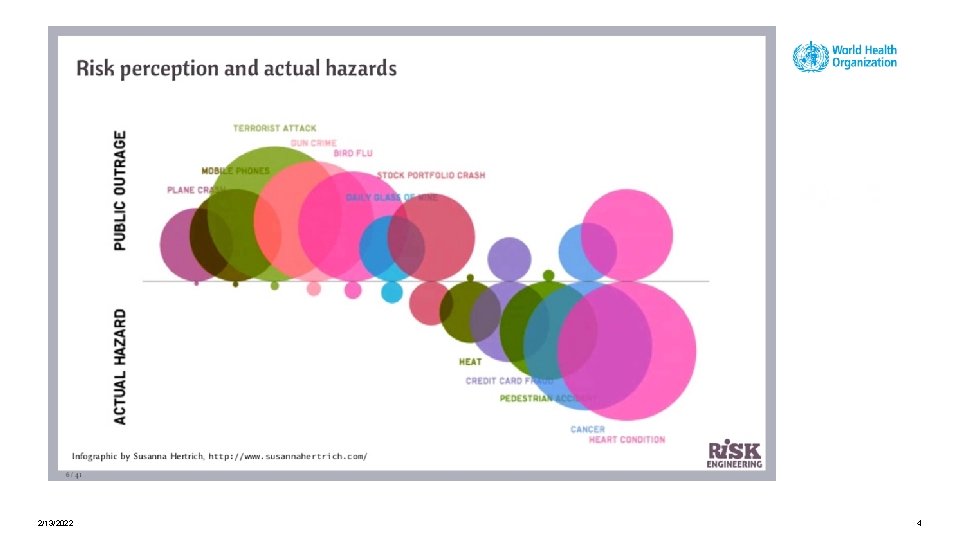
Risk perception is the subjective assessment of the probability of a specified type of accident happening and how concerned we are with the consequences. 2/13/2022 3
2/13/2022 4

The balance 2/13/2022 5
The balance Optimal Harm Benefit 2/13/2022 6

The balance 2/13/2022 7

The balance Benefit Harm 2/13/2022 8
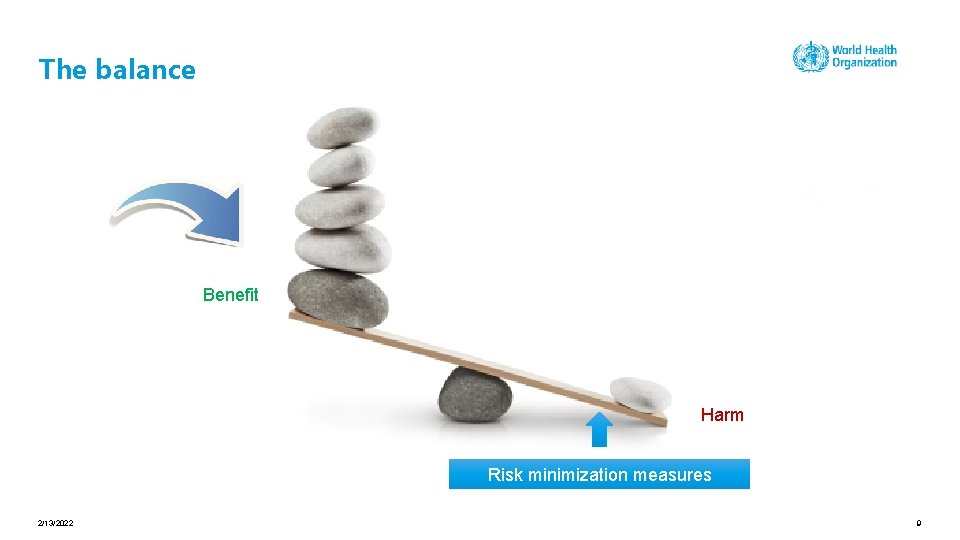
The balance Benefit Harm Risk minimization measures 2/13/2022 9

Risk minimisation measures Examples 2/13/2022 10
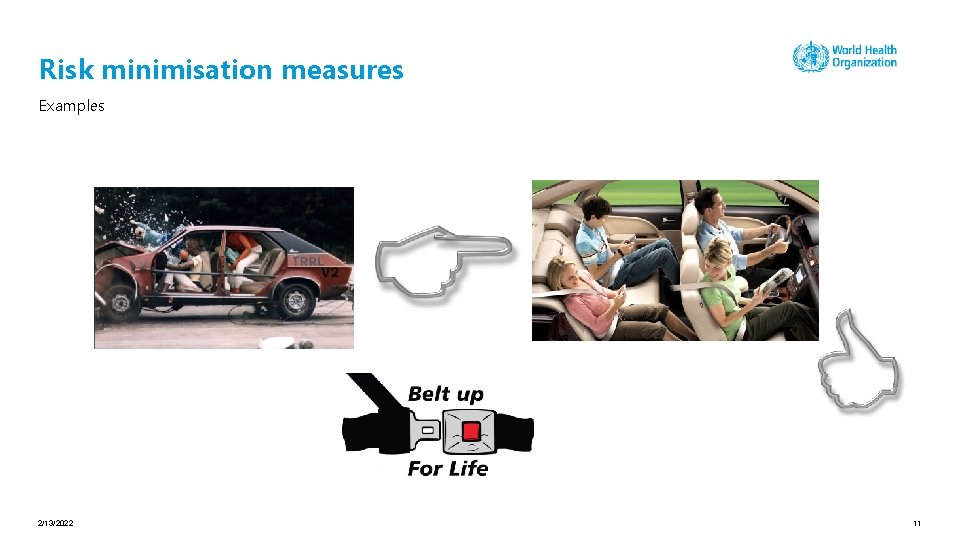
Risk minimisation measures Examples 2/13/2022 11
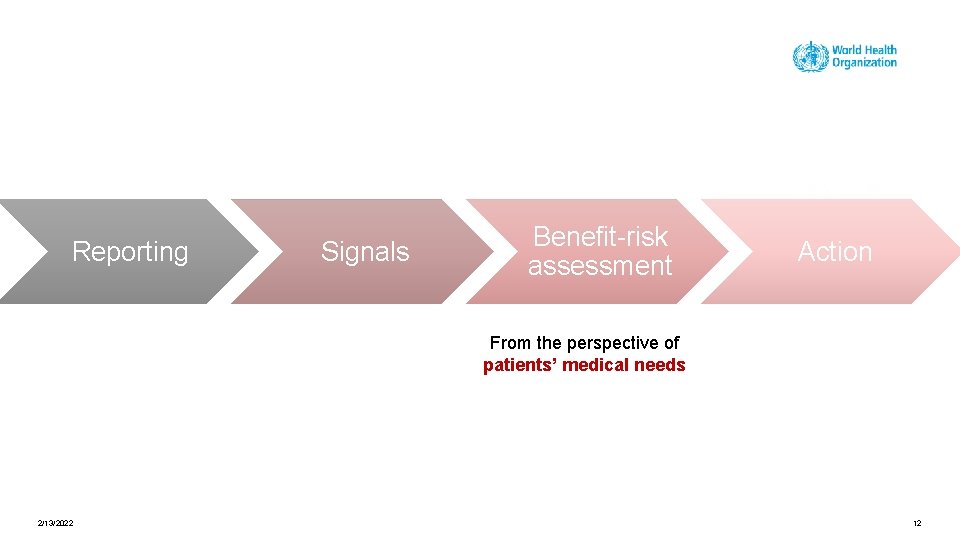
Reporting Signals Benefit-risk assessment Action From the perspective of patients’ medical needs 2/13/2022 12
Benefit vs. Harm Benefit/Risk assessment 2/13/2022 Benefit Harm Benefit/Risk assessment 13
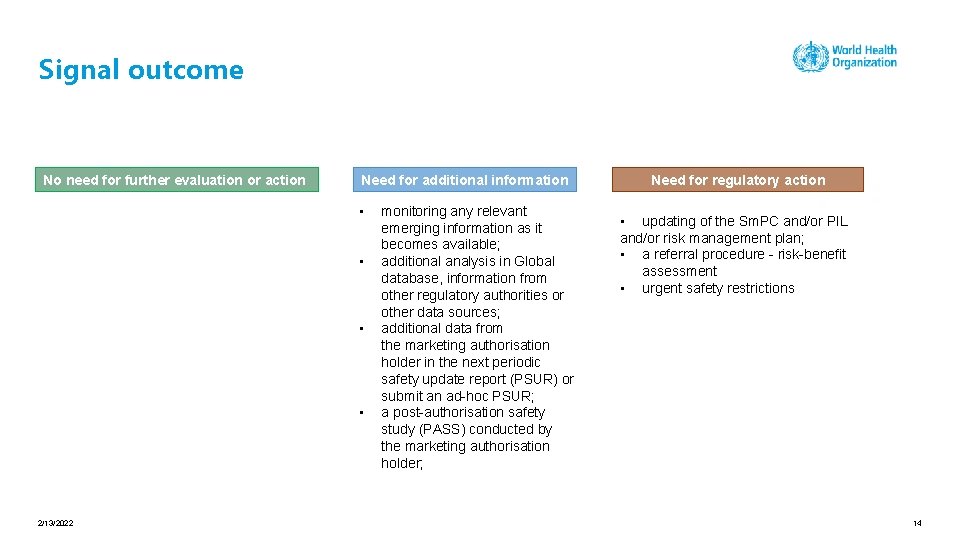
Signal outcome No need for further evaluation or action Need for additional information • • 2/13/2022 monitoring any relevant emerging information as it becomes available; additional analysis in Global database, information from other regulatory authorities or other data sources; additional data from the marketing authorisation holder in the next periodic safety update report (PSUR) or submit an ad-hoc PSUR; a post-authorisation safety study (PASS) conducted by the marketing authorisation holder; Need for regulatory action • updating of the Sm. PC and/or PIL and/or risk management plan; • a referral procedure - risk-benefit assessment • urgent safety restrictions 14
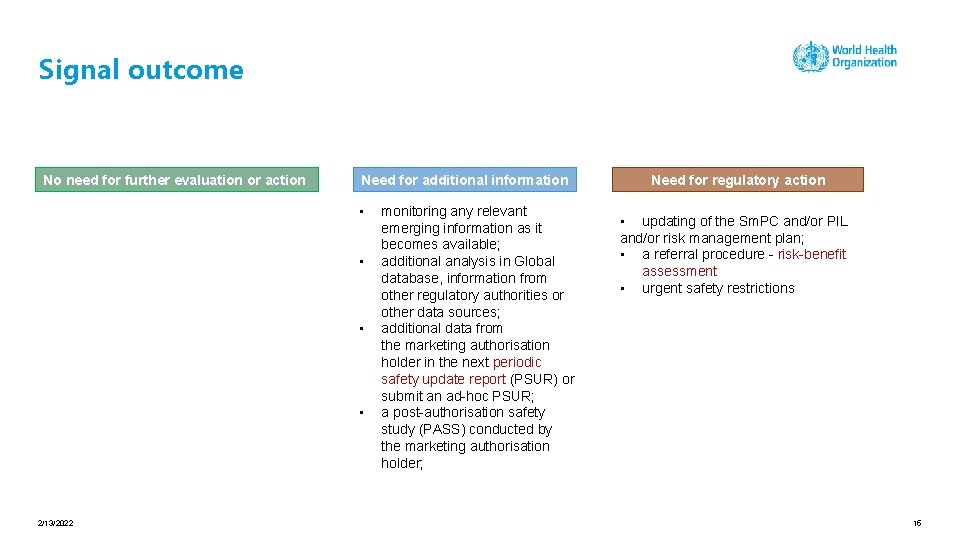
Signal outcome No need for further evaluation or action Need for additional information • • 2/13/2022 monitoring any relevant emerging information as it becomes available; additional analysis in Global database, information from other regulatory authorities or other data sources; additional data from the marketing authorisation holder in the next periodic safety update report (PSUR) or submit an ad-hoc PSUR; a post-authorisation safety study (PASS) conducted by the marketing authorisation holder; Need for regulatory action • updating of the Sm. PC and/or PIL and/or risk management plan; • a referral procedure - risk-benefit assessment • urgent safety restrictions 15

Benefit-risk assessment Questions which have to be asked– scope of the assessment Is it restricted to a drug substance or is it a therapeutic class safety issue? 2/13/2022 16

Benefit-risk assessment Questions which have to be asked– scope of the assessment Is it restricted to a drug substance or is it a therapeutic class safety issue? Is it limited to a specific indication, dosage, formulation, route of administration, or legal status? 2/13/2022 17

Benefit-risk assessment Questions which have to be asked– scope of the assessment Is it restricted to a drug substance or is it a therapeutic class safety issue? Is it limited to a specific indication, dosage, formulation, route of administration, or legal status? Should combination products be included? 2/13/2022 18

Benefit-risk assessment (cont. ) Questions which have to be asked– scope of the assessment Is it limited to specific patient(s) population – population at risk? 2/13/2022 19

Benefit-risk assessment (cont. ) Questions which have to be asked– scope of the assessment Is it limited to specific patient(s) population – population at risk? What is the specific clinical context of the safety concern(s) 2/13/2022 20

Benefit-risk assessment (cont. ) Questions which have to be asked– scope of the assessment Is it limited to specific patient(s) population – population at risk? What is the specific clinical context of the safety concern(s) Is there variability in how a therapy is used in clinical practice? 2/13/2022 21

Thalidomide 1960 s 2/13/2022 22
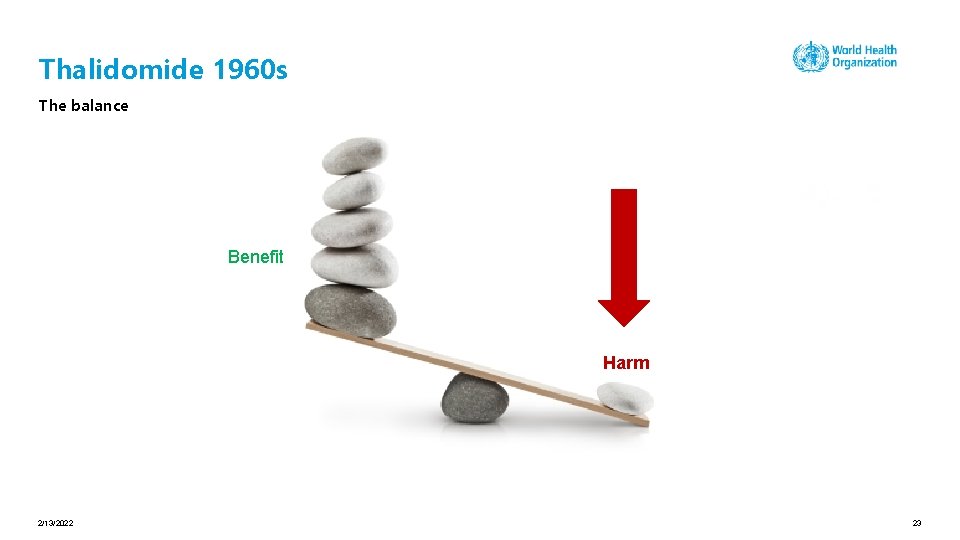
Thalidomide 1960 s The balance Benefit Harm 2/13/2022 23

Thalidomide 1960 s 1 6 9 1 n w a r d ith W 2/13/2022 24

Thalidomide in the 2000 s Reintroduction of a withdrawn medicine Risk management plan: • Pregnancy prevention plan • Restricted population • Controlled distribution • Package of educational and training tools 2/13/2022 25

Thalidomide 2000 s The balance Benefit Harm 2/13/2022 26
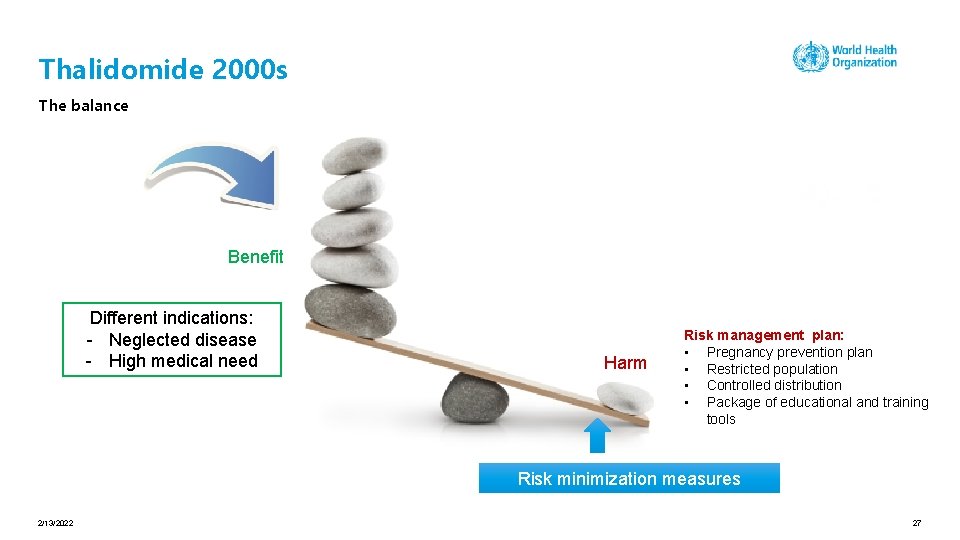
Thalidomide 2000 s The balance Benefit Different indications: - Neglected disease - High medical need Harm Risk management plan: • Pregnancy prevention plan • Restricted population • Controlled distribution • Package of educational and training tools Risk minimization measures 2/13/2022 27
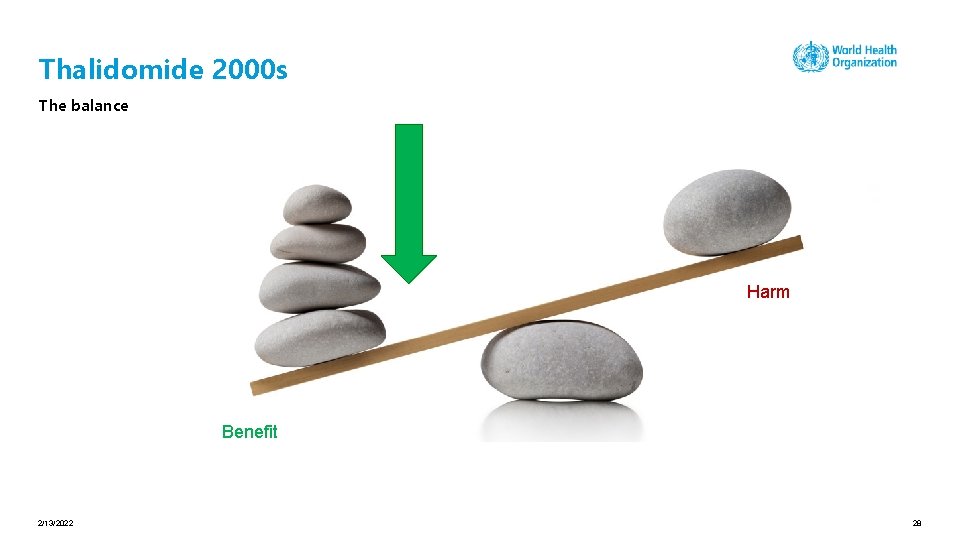
Thalidomide 2000 s The balance Harm Benefit 2/13/2022 28

Thalidomide in the 2000 s Reintroduction of a withdrawn medicine On the market with a risk management plan neglected disease and high medical need Risk management plan: • Pregnancy prevention plan • Restricted population • Controlled distribution • Package of educational and training tools 2/13/2022 29
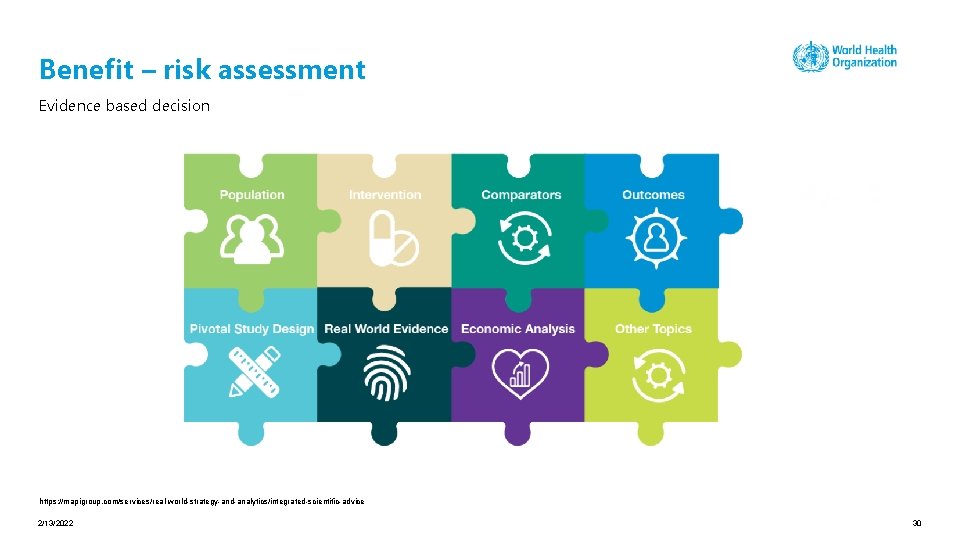
Benefit – risk assessment Evidence based decision https: //mapigroup. com/services/real-world-strategy-and-analytics/integrated-scientific-advice 2/13/2022 30
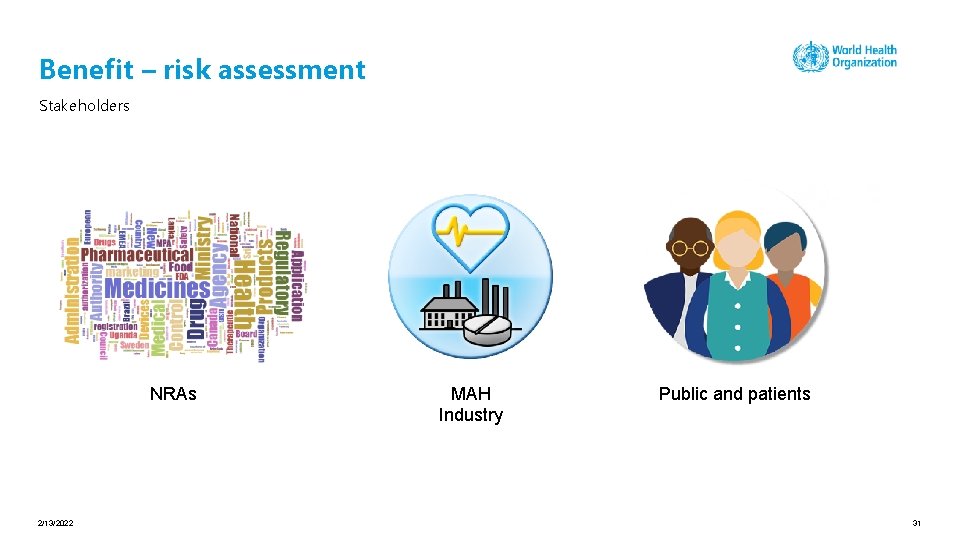
Benefit – risk assessment Stakeholders NRAs 2/13/2022 MAH Industry Public and patients 31

Benefit – risk assessment Stakeholders Public health programmes 2/13/2022 32

Advisory safety committee Pool of experts – PV, regulatory, clinical, patient safety, biostatisticians, epidemiologists, public health program… 2/13/2022 33

Benefit – risk assessment What to present to a Safety advisory committee Background: the safety concern and scope; Overview of the key benefits and risks; Summary of evidence relating to the safety concern under consideration; Conclusion on the evidence; Options and rationale for regulatory action - advantages and disadvantages; Need for risk minimisation measures; Expert opinion; MAH(s) position; Modified from SCOPE Joint action 2013 -2016, WP 8 2/13/2022 34

Benefit – risk assessment (cont. ) What to present to a Safety advisory committee Final conclusions and recommendations including current Patient Information wording (if any) and proposed updates and/or any RMP amendments List of outstanding issues including the need for further studies (Non-interventional studies for example) Communication plan Key areas for discussion Modified from SCOPE Joint action 2013 -2016, WP 8 2/13/2022 35
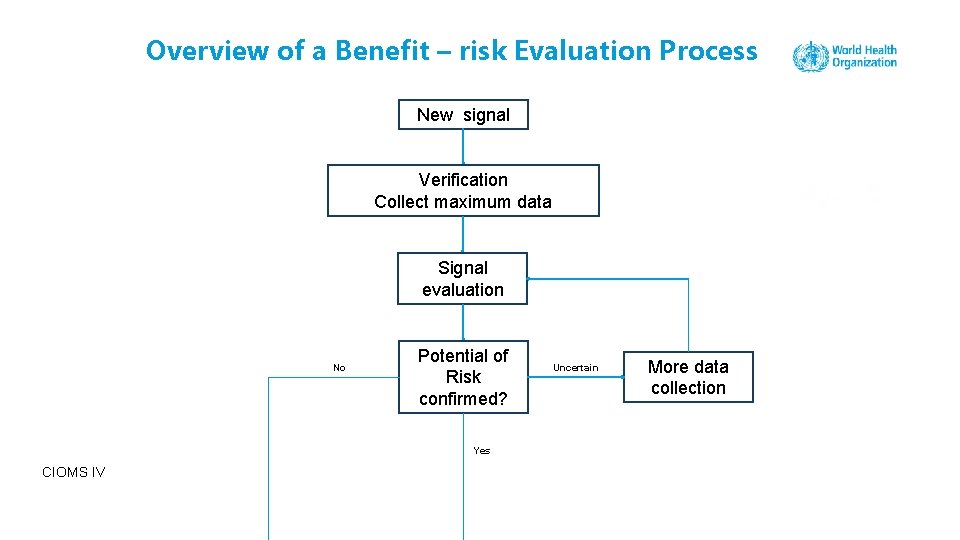
Overview of a Benefit – risk Evaluation Process New signal Verification Collect maximum data Signal evaluation No Potential of Risk confirmed? Uncertain More data collection Yes CIOMS IV Tutorial Benefit – risk assessment 36
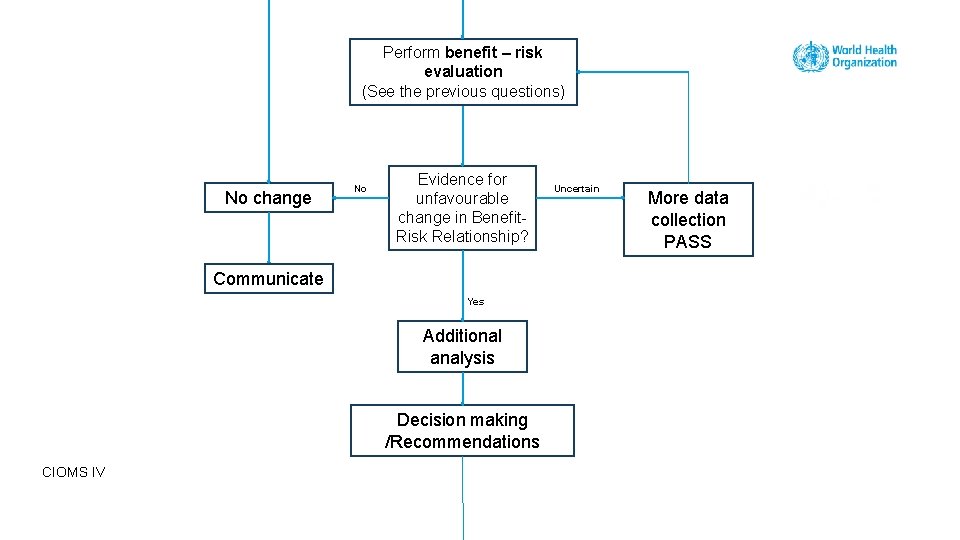
Perform benefit – risk evaluation (See the previous questions) No change No Evidence for unfavourable change in Benefit. Risk Relationship? Uncertain More data collection PASS Communicate Yes Additional analysis Decision making /Recommendations CIOMS IV Tutorial Benefit – risk assessment 37
Communicate Implement Monitor the impact of the recommendations CIOMS IV Tutorial Benefit – risk assessment 38
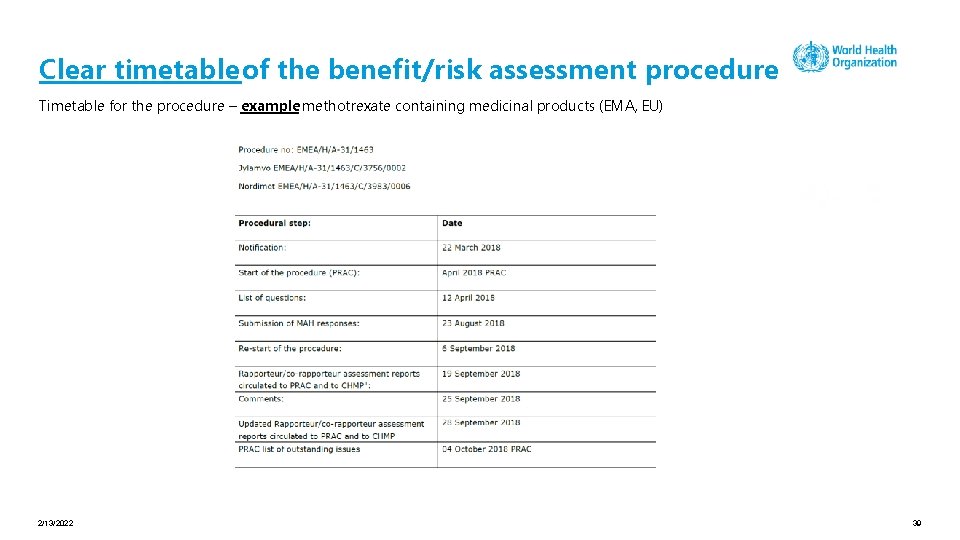
Clear timetable of the benefit/risk assessment procedure Timetable for the procedure – example methotrexate containing medicinal products (EMA, EU) 2/13/2022 39
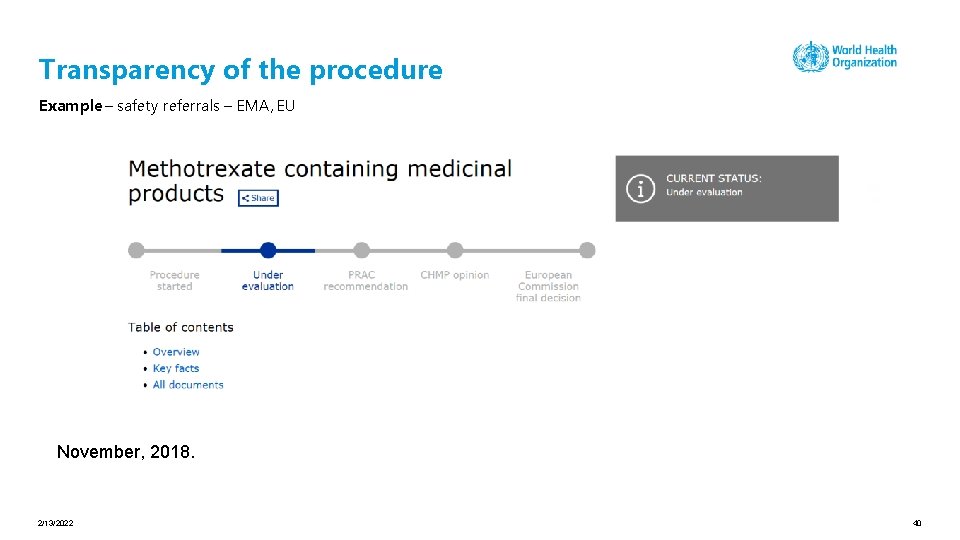
Transparency of the procedure Example – safety referrals – EMA, EU November, 2018. 2/13/2022 40
CIOMS IV 2/13/2022 41
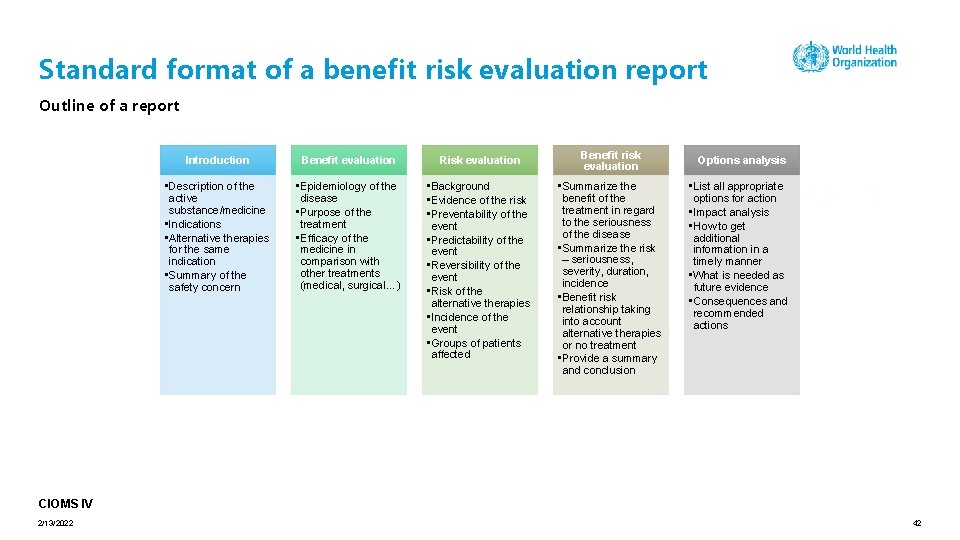
Standard format of a benefit risk evaluation report Outline of a report Introduction Benefit evaluation Risk evaluation • Description of the active substance/medicine • Indications • Alternative therapies for the same indication • Summary of the safety concern • Epidemiology of the disease • Purpose of the treatment • Efficacy of the medicine in comparison with other treatments (medical, surgical…) • Background • Evidence of the risk • Preventability of the event • Predictability of the event • Reversibility of the event • Risk of the alternative therapies • Incidence of the event • Groups of patients affected Benefit risk evaluation • Summarize the benefit of the treatment in regard to the seriousness of the disease • Summarize the risk – seriousness, severity, duration, incidence • Benefit risk relationship taking into account alternative therapies or no treatment • Provide a summary and conclusion Options analysis • List all appropriate options for action • Impact analysis • How to get additional information in a timely manner • What is needed as future evidence • Consequences and recommended actions CIOMS IV 2/13/2022 42
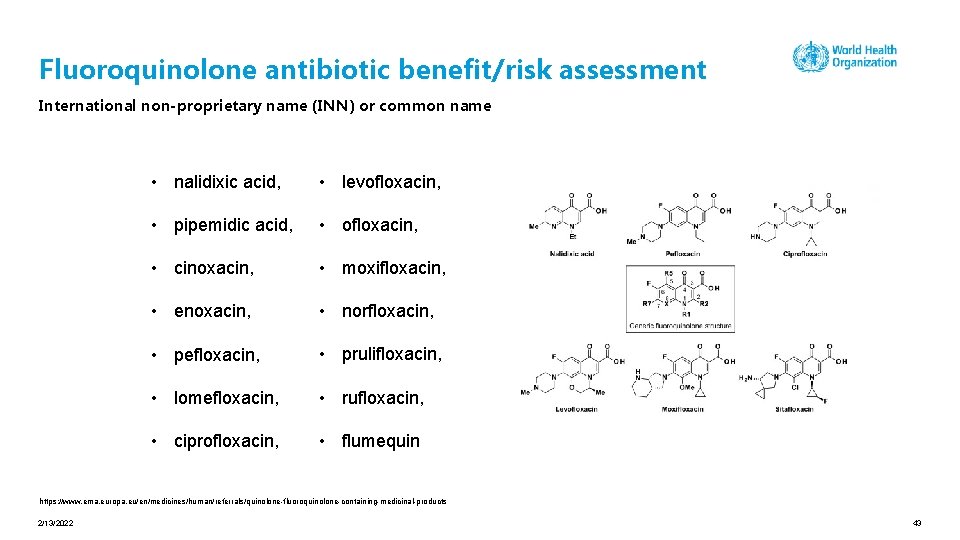
Fluoroquinolone antibiotic benefit/risk assessment International non-proprietary name (INN) or common name • nalidixic acid, • levofloxacin, • pipemidic acid, • ofloxacin, • cinoxacin, • moxifloxacin, • enoxacin, • norfloxacin, • pefloxacin, • prulifloxacin, • lomefloxacin, • rufloxacin, • ciprofloxacin, • flumequin https: //www. ema. europa. eu/en/medicines/human/referrals/quinolone-fluoroquinolone-containing-medicinal-products 2/13/2022 43

Fluoroquinolone antibiotic benefit/risk assessment Signal confirmed as adverse drug reaction– class effect of fluoroquinolones Serious adverse drug reactions (ADRs) include (as a syndrome): • • • 2/13/2022 inflamed or torn tendon, muscle pain or weakness, joint pain or swelling, walking difficulty, feeling pins and needles (polyneuropathy), burning pain, tiredness, depression, problems with memory, sleeping, vision and hearing, and altered taste and smell. 44
Fluoroquinolone antibiotic benefit/risk assessment Signal confirmed as adverse drug reaction– class effect of fluoroquinolones (cont. ) Tendon swelling and injury may occur within 2 days of starting treatment with a fluoroquinolone but may even occur several months after stopping treatment 2/13/2022 45
Fluoroquinolone antibiotic benefit/risk assessment The review Started 9 February 2017 The review incorporated the views of: patients, healthcare professionals and academics presented at EMA’s public hearing on fluoroquinolone and quinolone antibiotics in June 2018. 2/13/2022 46

Fluoroquinolone antibiotic benefit/risk assessment The review – the list of questions Question 1 Provide any available evidence pointing to long-lasting, disabling and potentially irreversible adverse drug reactions (for example musculoskeletal impairment, peripheral neuropathy, neurological or psychiatric reactions, impairment of the senses) associated with the use of your product(s), including a review and discussion of clinical and epidemiological studies and literature relating to your product(s). As applicable, the methodology used should be provided. 2/13/2022 47
Fluoroquinolone antibiotic benefit/risk assessment The review – the list of questions Question 2 Please discuss possible mechanisms of long lasting, disabling and potentially irreversible adverse drug reactions, including a discussion on vasculitis, effects on mitochondria and mt. DNA, effects on oxidative stress, tendinocytes and matrix metalloproteinases, based on non -clinical and clinical published and unpublished data for your product(s). 2/13/2022 48

Fluoroquinolone antibiotic benefit/risk assessment The review – the list of questions Question 3 Please provide an assessment on the impact of occurrence of long lasting, disabling and potentially irreversible adverse drug reactions on the benefit-risk balance of your product(s) in the currently approved indication(s) in the EU. This assessment should take into account the characteristics of the clinical condition(s) for which the product is indicated. 2/13/2022 49

Fluoroquinolone antibiotic benefit/risk assessment The review – the list of questions Question 4 Provide proposals and justifications with supportive evidence for any RMMs (including changes to the Sm. PC/PL) that may improve the benefit/risk balance of your product(s), and how their effectiveness should be monitored. Please discuss whether additional RMMs (e. g. DHPC) are warranted in view of the above. In case a DHPC is considered warranted a DHPC proposal including a proposal for a communication plan should be provided. 2/13/2022 50
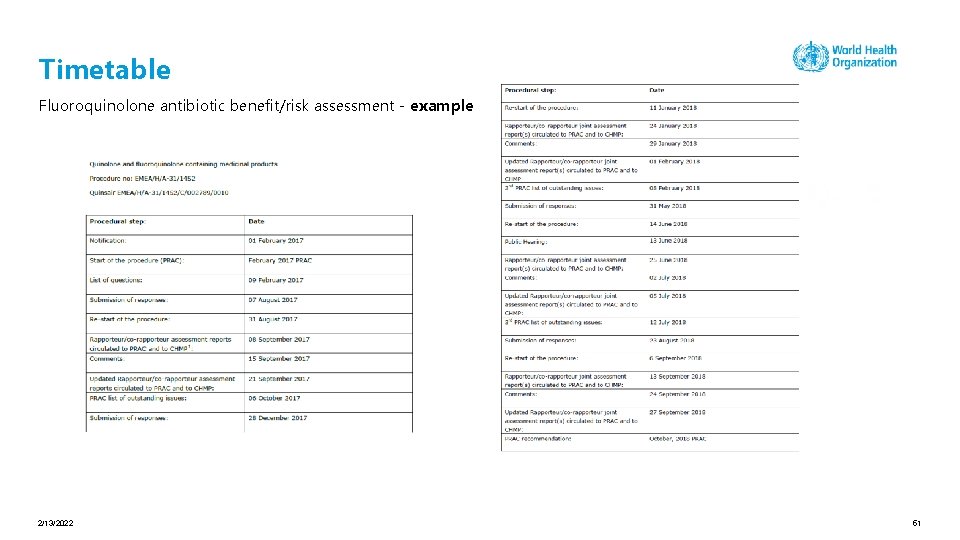
Timetable Fluoroquinolone antibiotic benefit/risk assessment - example 2/13/2022 51

Fluoroquinolone antibiotic benefit/risk assessment Recommendations – October 2018 The marketing authorization of medicines containing cinoxacin, flumequine, nalidixic acid, and pipemidic acid should be suspended in the European Union. https: //www. ema. europa. eu/documents/referral/quinolone-fluoroquinolone-article-31 -referral-disabling-potentially-permanent-side-effects-lead_en. pdf 2/13/2022 52
Fluoroquinolone antibiotic benefit/risk assessment Recommendations – October 2018 The use of the remaining fluoroquinolone antibiotics should be restricted https: //www. ema. europa. eu/documents/referral/quinolone-fluoroquinolone-article-31 -referral-disabling-potentially-permanent-side-effects-lead_en. pdf 2/13/2022 53

Fluoroquinolone antibiotic benefit/risk assessment Recommendations – October 2018 Fluoroquinolone antibiotics should not be used: used • to treat infections that might get better without treatment or are not severe (such as throat infections); • to treat non-bacterial infections, e. g. non-bacterial (chronic) prostatitis; • for preventing traveler's diarrhea or recurring lower urinary tract infections • to treat mild or moderate bacterial infections unless other antibacterial medicines commonly recommended for these infections cannot be used. https: //www. ema. europa. eu/documents/referral/quinolone-fluoroquinolone-article-31 -referral-disabling-potentially-permanent-side-effects-lead_en. pdf 2/13/2022 54
Fluoroquinolone antibiotic benefit/risk assessment Recommendations Fluoroquinolones should generally be avoided in patients who have previously had serious side effects with a fluoroquinolone or quinolone antibiotic. They should be used with special caution in the elderly, patients with kidney disease and those who have had an organ transplantation because these patients are at a higher risk of tendon injury. Since the use of a corticosteroid with a fluoroquinolone also increases this risk, combined use of these medicines should be avoided. https: //www. ema. europa. eu/documents/referral/quinolone-fluoroquinolone-article-31 -referral-disabling-potentially-permanent-side-effects-lead_en. pdf 2/13/2022 55
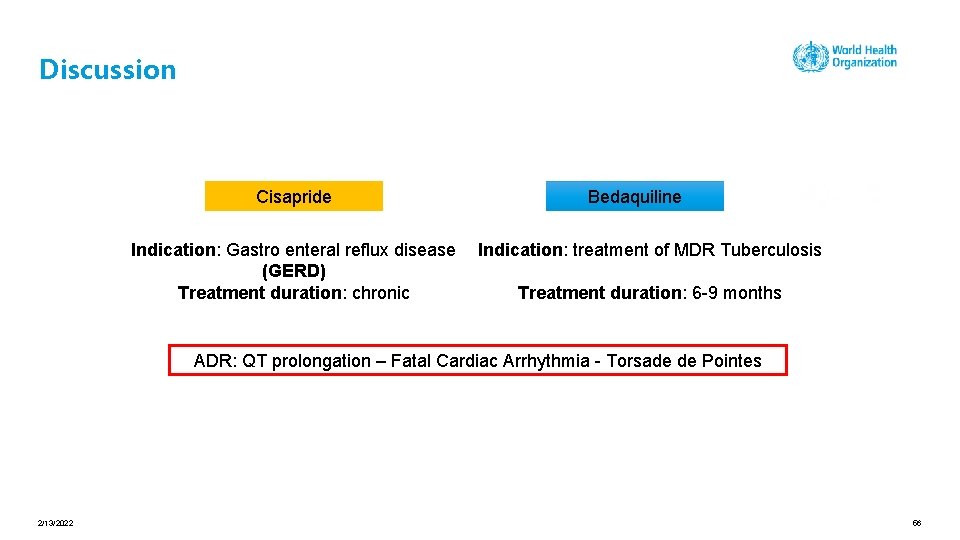
Discussion Cisapride Indication: Gastro enteral reflux disease (GERD) Treatment duration: chronic Bedaquiline Indication: treatment of MDR Tuberculosis Treatment duration: 6 -9 months ADR: QT prolongation – Fatal Cardiac Arrhythmia - Torsade de Pointes 2/13/2022 56

Thank you very much! Reading materials: https: //cioms. ch/wp-content/uploads/2017/01/benefit-risk. pdf http: //www. scopejointaction. eu/downloads/scope-wp 8 lifecycle-pharmacovigilance/ Tutorial Benefit – risk assessment 57
- Slides: 57