Benchmark dose methodology for setting human health regulatory

Benchmark dose methodology for setting human health regulatory endpoints Dr Nick Fletcher

Introduction • EFSA Benchmark dose workshop, 1 -2 March, 2017 • EFSA, EC, EMA, US EPA, US FDA, FSANZ, Food Safety Commission Japan, WHO, other international agencies and bodies • EFSA updated guidance and released a new software platform
Scope • Focus will be on the utility of dose response models and BMD in risk assessment • Only limited treatment of the mathematical and statistical considerations for BMD modelling • Example of the application of BMD to a processing contaminant (workshop) using available software
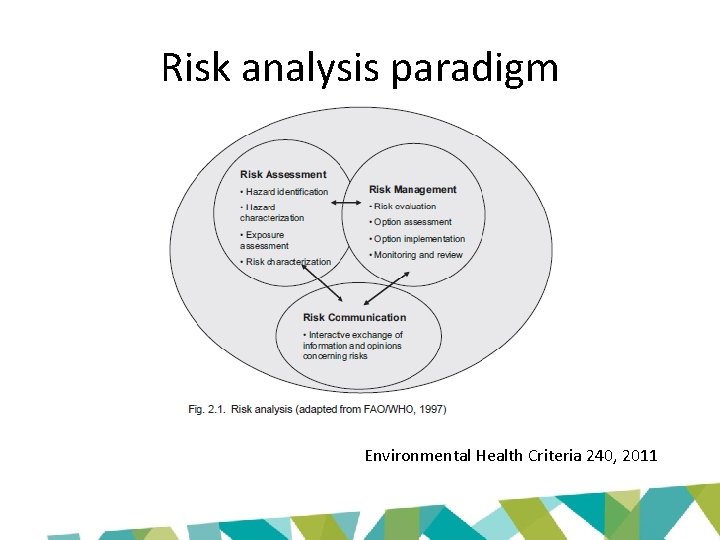
Risk analysis paradigm Environmental Health Criteria 240, 2011
Dose response assessment in hazard characterisation • NOAEL typically used to establish HBGVs such as ADI or TDI for non-genotoxic substances • Proposed to use BMD approach to obtain a POD on dose response curve and calculate a MOE for genotoxic and carcinogenic substances • More recent guidance concluded BMD also appropriate for non-genotoxic substances

Typically involves two statistical approaches • ANOVA and pairwise comparisons to define the NOAEL or LOAEL • Fit a model or models to the dose-response data to define the relationship [within or slightly below the observed experimental range]
What is the NOAEL approach? • Highest concentration/amount of substance that produces no adverse effects • Toxicity tests generally detect biological responses in the range of 5 -10% • HBGV = NOAEL / Uncertainty factors

Advantages of NOAEL approach • Long history of use in regulating a range of chemicals • OECD Testing guidelines developed to ensure that toxicological studies define a NOAEL • On average gives the same result as the BMD approach – less resources • Easy to communicate
Arguments against a NOAEL approach • More dependent upon the dose levels and dose spacing selected in the study • Does not consider all of the available information • Rewards studies with low power (eg small group sizes) as these tend to result in higher NOAELs • Argued that the terminology does not recognise that these are actually effect levels
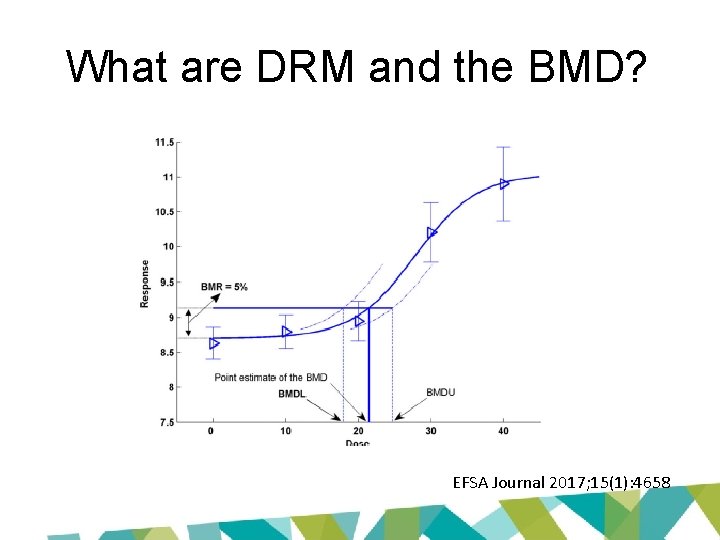
What are DRM and the BMD? EFSA Journal 2017; 15(1): 4658

Advantages • Not limited to experimental doses • Less dependent on dose spacing • Takes into account the shape of doseresponse level • Takes account of variability and uncertainty arising from study quality • Under some circumstances, can be used to compare results across chemicals and studies
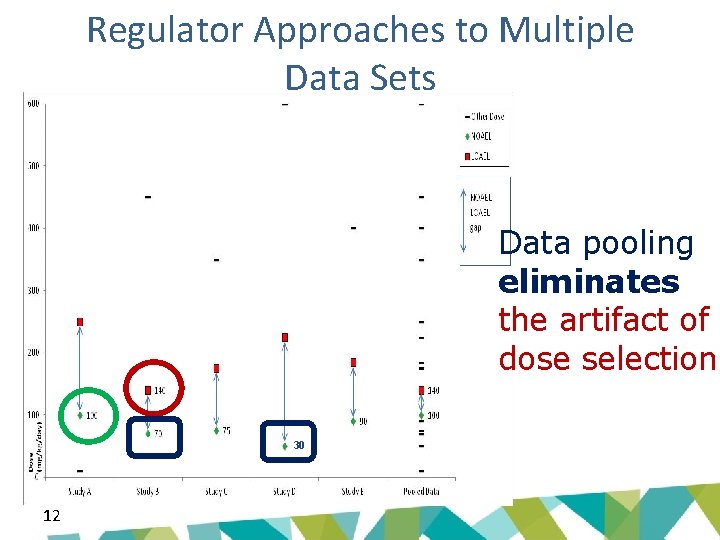
Regulator Approaches to Multiple Data Sets Data pooling eliminates the artifact of dose selection 30 12

Disadvantages • Complexity – Requires a certain quality and quantity of data – Resource intensive and requires specific expertise – More complicated decision making process – can be inconsistent between different agencies – And gives ‘on average’ the same result as the NOAEL approach – More difficult to communicate
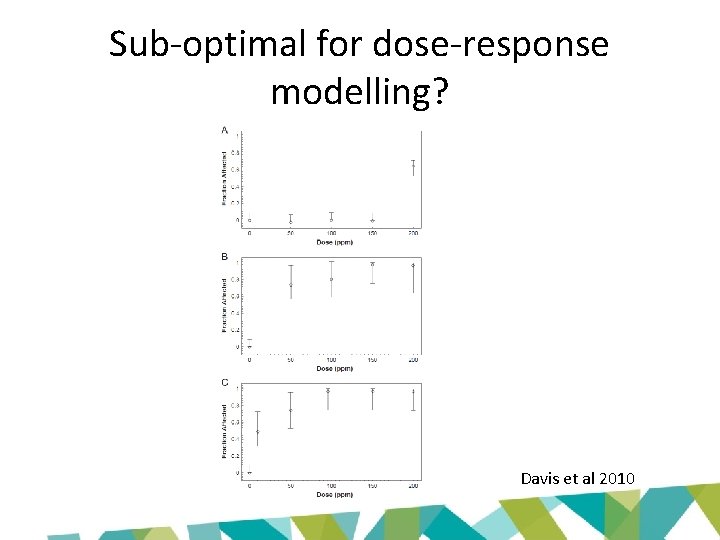
Sub-optimal for dose-response modelling? Davis et al 2010
What are the steps in generating a BMD? • Determine the type of data (eg continuous; quantal) • Specification of a response level (BMR) • Selection of candidate dose-response models • Fitting a set of dose–response models resulting in a set of BMD confidence intervals • Deriving a single BMD confidence interval for that particular adverse effect/endpoint, preferably by model averaging
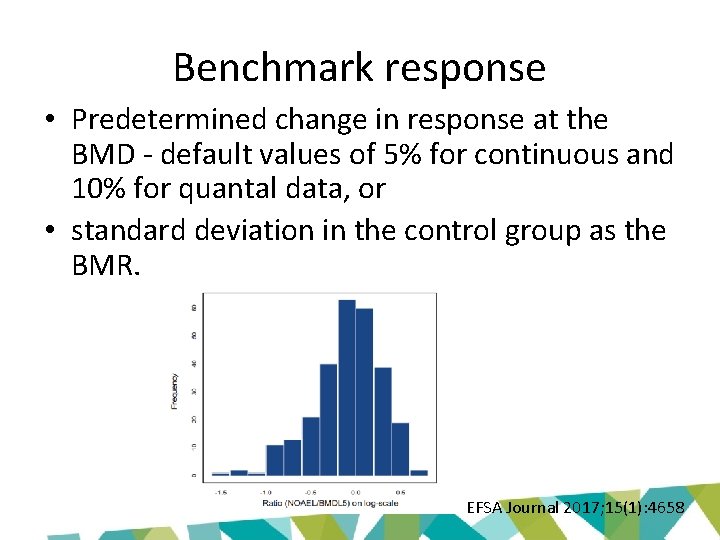
Benchmark response • Predetermined change in response at the BMD - default values of 5% for continuous and 10% for quantal data, or • standard deviation in the control group as the BMR. EFSA Journal 2017; 15(1): 4658

Running the models • Benchmark dose software (BMDS) developed by the US EPA (www. epa. gov/bmds) • PROAST software developed by RIVM (www. rivm. nl/proast). • EFSA software at https: //efsa. openanalytics. eu/login
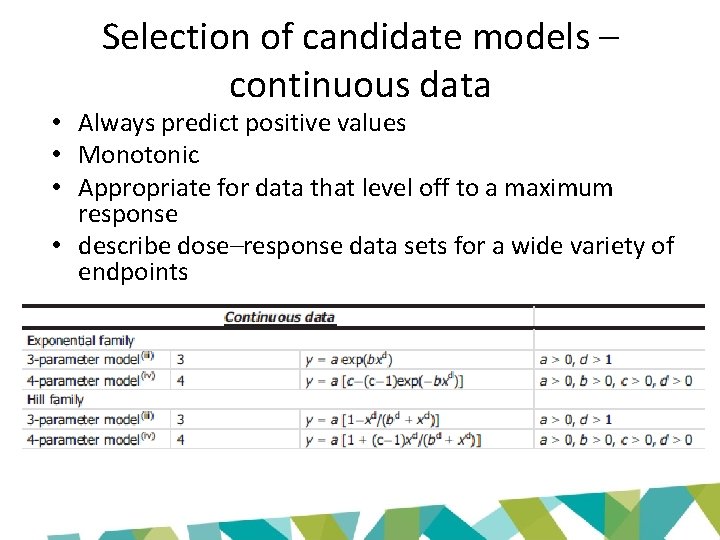
Selection of candidate models – continuous data • Always predict positive values • Monotonic • Appropriate for data that level off to a maximum response • describe dose–response data sets for a wide variety of endpoints
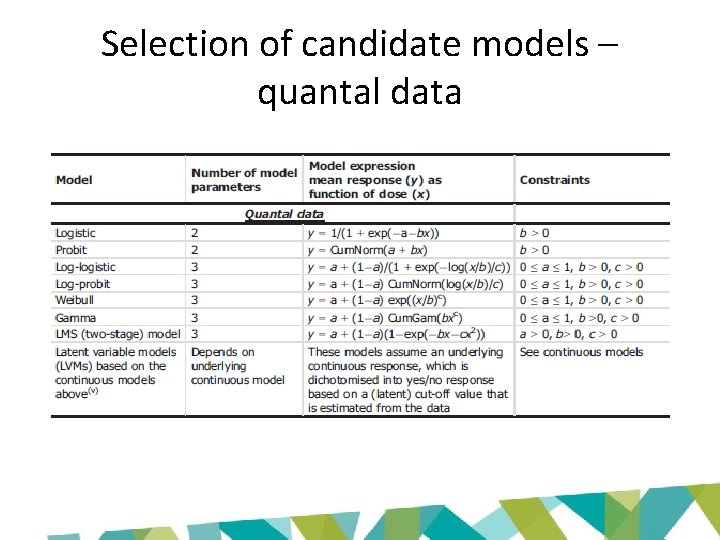
Selection of candidate models – quantal data
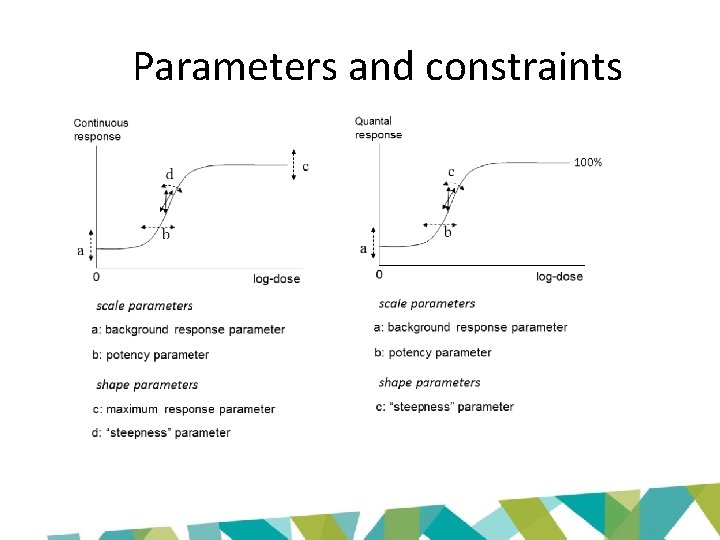
Parameters and constraints
Model evaluation • Fitting the models achieved by software • In BMDS and PROAST has been achieved by maximising the log-likelihood • EFSA recommends use of the AIC criterion – AIC should be lower than AICnull-2 – AIC should be no more than two units higher than the full model
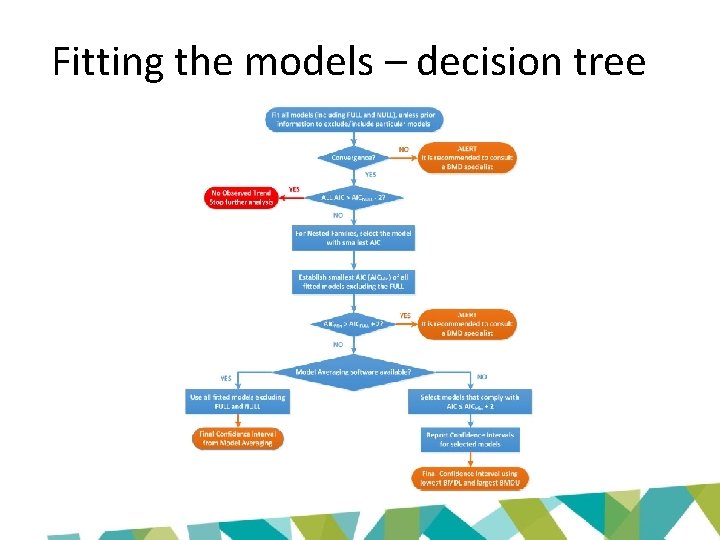
Fitting the models – decision tree
What is model averaging? • Model averaging attempts to take into account model uncertainty by incorporating information from all models • Individual models are combined using weights – defined by AIC • BMD is estimated at BMR. The BMDL and BMDU are estimated via parametric bootstrapping
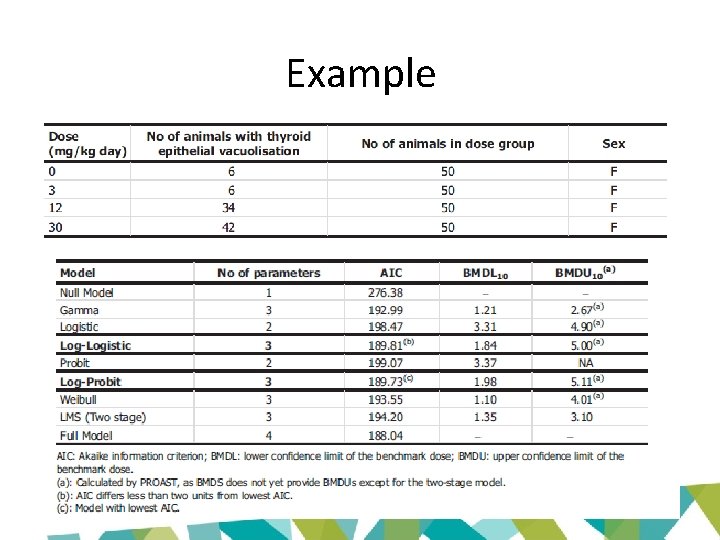
Example
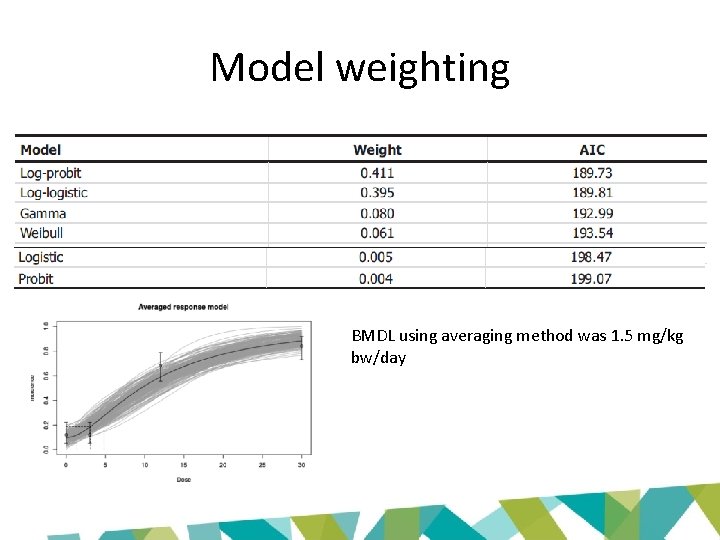
Model weighting BMDL using averaging method was 1. 5 mg/kg bw/day
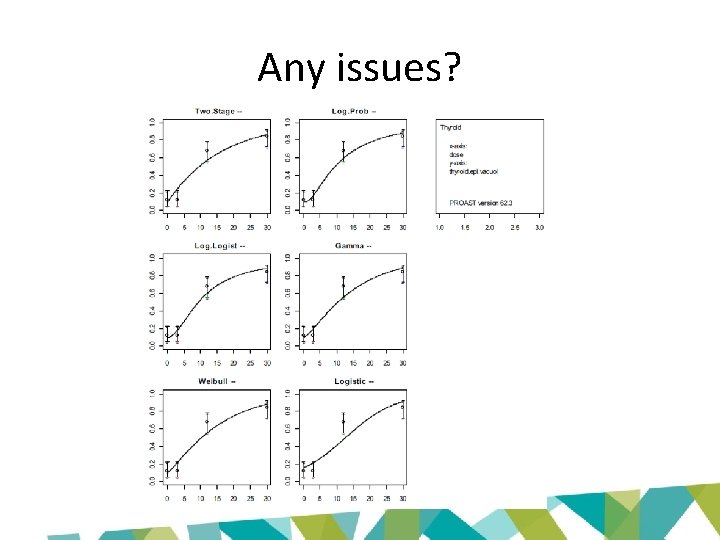
Any issues?
Scientific judgment • BMD models may improve and generate more reliable predictions but they can never be proved to be completely correct • Necessary to rely on scientific judgement to determine utility of risk predictions in making public health decisions • The final standard that prevails in risk assessment is biological plausibility

Conclusions • EFSA, US EPA and WHO committed to using BMD modelling where appropriate • Updated guidance and new (user friendly) software available • To optimise would require changes to tox testing protocols • Better understanding of how and when to define BMR – what to do at low doses • Aim to harmonise international approaches where possible but work still to be done

Everyone seems to agree– but are there still problems? • JECFA POD for 3 -MCPD is an order of magnitude greater than the value chosen by EFSA using the same data and same model suite, – Why? – Which one is correct? – Will model averaging give another result? – If so which one do use? – Should we extrapolate outside the experimental range?
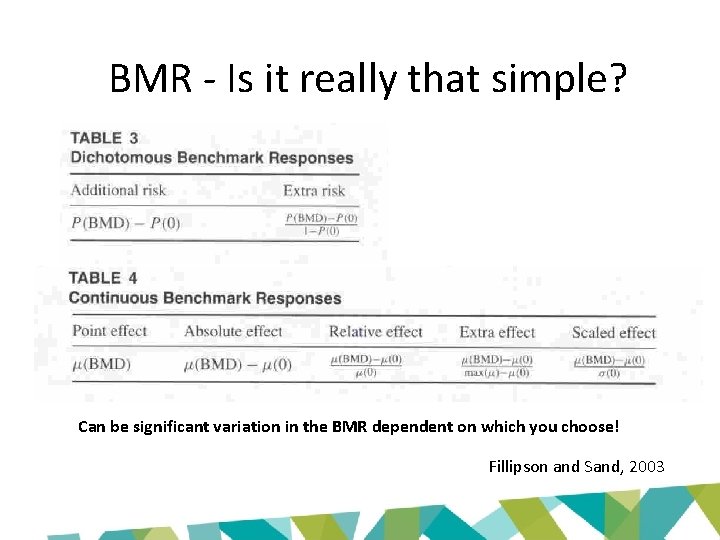
BMR - Is it really that simple? Can be significant variation in the BMR dependent on which you choose! Fillipson and Sand, 2003

Copyright © Food Standards Australia New Zealand 2017 This work is copyright. You may download, display, print and reproduce this material in unaltered form only (retaining this notice) for your personal, non-commercial use or use within your organisation. Apart from any other use as permitted under the Copyright Act 1968, all other rights are reserved. Requests for further authorisation should be directed to information@foodstandards. gov. au www. foodstandards. gov. au or www. foodstandards. govt. nz /Food. Standards @FSANZnews
- Slides: 31