Bellringer Classify the following as an element compound

Bellringer • Classify the following as an element, compound, homogeneous mixture, or heterogeneous mixture. – Granite – Diamond – Gatorade – Rust • Classify the following as physical or chemical changes. – Water is heated and turns to steam – A rock is broken apart by weathering – Grass grows on a lawn • Don’t forget I need you to submit the assignment on Google Classroom by TONIGHT (Tyree, Emily, Jayla, Sydney, Sharon, Meredith, Ryan, Maddie, Chloe, Jocelyne, Natasha, Julia, Fabienne, Shane, Emeline, Payten – have done)

Minerals

Minerals • • • 5 characteristics of a mineral – Naturally-occurring Inorganic Solid substance Specific chemical composition Crystalline structure

Minerals • Naturally-occurring – formed by natural processes, not man-made • Quartz is found naturally, so it is a mineral • Cubic zirconium is man-made, so it is NOT a mineral Quartz Cubic Zirconium

Minerals • Inorganic – not formed by anything that was ever alive • Salt is a mineral, while sugar (which comes from plants) is not • Coal (which comes from fossilized plants) is not a mineral

Minerals • Solid -- has a definite shape and volume

Minerals • Specific chemical composition – made of the same elements/compounds each time • Some minerals (ex: copper, sulfur, gold) are made of single elements • Most, however, are made of compounds (elements bonded together) • Example: quartz (Si. O 2) always consists of one silicon bonded to two oxygen atoms Quartz

Minerals • Granite is made of a variety of minerals, so it does not always have the same chemical composition • Granite is a rock (NOT a mineral)
Minerals • Crystalline structure – atoms in minerals are arranged in repeating geometric patterns • This structure is found in every mineral on an atomic level

• Crystalline habit – the external expression of a mineral’s internal crystalline structure • Minerals must grow without interference or obstacle in order to outwardly show their crystalline shape • Examples: Salt will form cubes; Quartz forms hexagons
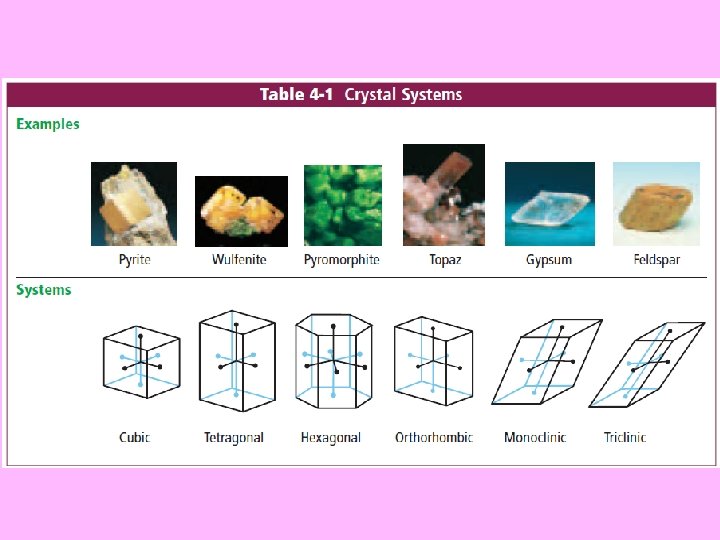

Minerals • There are over 3000 minerals in Earth’s crust, but only about 30 are common • The most common of those 30 are called rockforming minerals
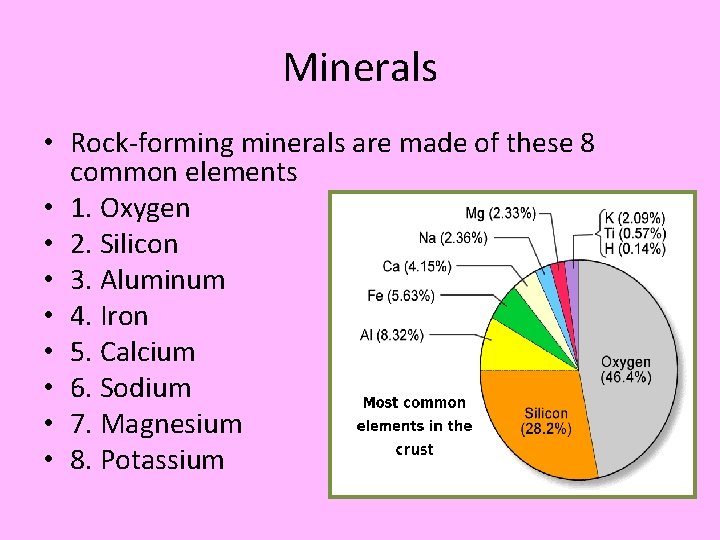
Minerals • Rock-forming minerals are made of these 8 common elements • 1. Oxygen • 2. Silicon • 3. Aluminum • 4. Iron • 5. Calcium • 6. Sodium • 7. Magnesium • 8. Potassium

Mineral Formation • • Minerals can form in a variety of ways Cooling Magma Precipitation Evaporation

Mineral Formation • Cooling Magma • Magma -- hot, molten rock under the Earth’s crust • Magma cools more slowly than lava Above the surface = lava Below the surface = magma

Mineral Formation • The type and amount of elements present in the magma determine what minerals will form • The rate at which magma cools determines the size of the mineral crystals • Cooling slowly forms large crystals • Cooling quickly forms small crystals Large crystals Small crystals No crystals

Mineral Formation • Precipitation • When liquids become supersaturated with dissolved solids, mineral crystals begin to precipitate, or drop out of solution • Example: this is how the rock limestone (made from the mineral calcite) is formed

Mineral Formation • Evaporation • When water evaporates, the dissolved solids are left behind and may arrange into minerals • Example: salt flats in deserts

Mineral Groups • Classified based on the elements that make them up • 7 Main Groups

Mineral Groups • Silicates – minerals that contain the elements silicon and oxygen (and usually one or more other elements) • Most common mineral group (since Si & O are most abundant elements in Earth’s crust) • Examples: feldspar, quartz

Mineral Groups • Carbonates -- minerals composed of at least one element bonded to a carbonate compound (CO 3 -2) • Second most common mineral group • Example: Calcite (Ca. CO 3)

Mineral Groups • Oxides – composed of oxygen and a metal • Examples: Hematite (Fe 2 O 3) and Magnetite (Fe 3 O 4) • The mineral uraninite is valuable because it is the source of uranium, used in nuclear power plants

Mineral Groups • Other Groups: • Sulfides – contain sulfur (ex: pyrite) • Sulfates – contain sulfate (SO 4 -2) (ex: gypsum) pyrite gypsum

Mineral Groups • Other Groups: • Halides – contain chlorine or fluorine (halogens) along with Ca, Na, or K halite • Ex: halite (salt) • Native Elements – pure elements that occur by themselves • Ex: silver (Ag), copper (Cu), (C), graphite (C) diamonds graphite diamond

Mineral Groups • Ores – any minerals that contains a useful substance that can be mined for a profit • Ores found near Earth’s surface are mined in open-pit mines Bauxite (ore) is mined for its aluminum content

Mineral Groups • Gemstones -- beautiful, rare, valuable minerals that are cut and polished for jewelry.

Identifying Minerals • Minerals are identified based on their physical properties • Color -- the most obvious property of a mineral, also the least reliable • Small amounts of impurities can give the same mineral different colors Yellow, blue, and red… all the same mineral (sapphire)

Identifying Minerals • Luster – how light is reflected off a mineral’s surface • Metallic – shiny like gold or silver • Nonmetallic – pearly, vitreous (glassy), resinous (resin-like), silky, earthy (dull)

Identifying Minerals • Texture -- how the mineral feels • Examples: smooth, rough, greasy, glassy, etc.

Identifying Minerals • Streak – the color of a mineral in its powdered form • The mineral is rubbed on the back of a porcelain tile • The streak color can be different than the mineral’s color • Example: gold and fool’s gold

Identifying Minerals • Hardness -- resistance of a mineral to being scratched

Identifying Minerals • Ranked on the Mohs hardness scale • Softest – Talc, 1, all other minerals scratch it • Hardest – Diamond, 10, scratches all other Moh’s hardness scale minerals Higher numbered minerals scratch lower numbered minerals.

Identifying Minerals • Cleavage – when a • Fracture – when a mineral cleaves (breaks) mineral breaks with evenly along a plane rough, jagged edges • Examples: calcite, mica
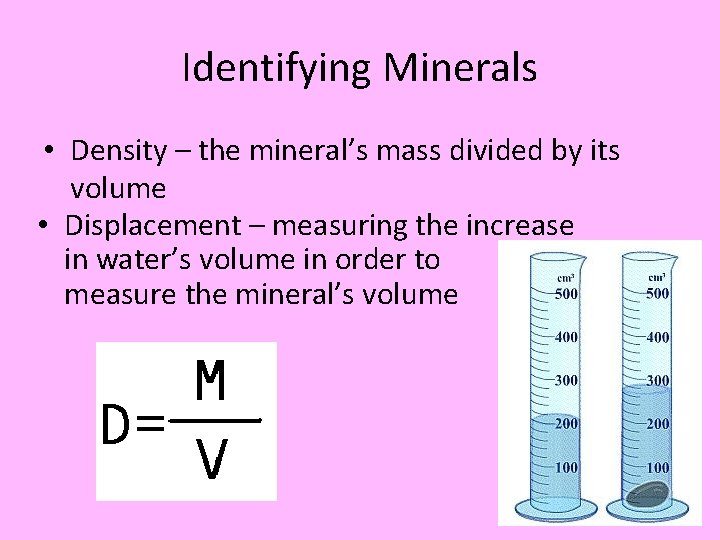
Identifying Minerals • Density – the mineral’s mass divided by its volume • Displacement – measuring the increase in water’s volume in order to measure the mineral’s volume

Identifying Minerals • Some minerals have special properties • Magnetite attracts to magnets • Calcium carbonate bubbles with the addition of acid (effervescence) • Sulfur smells like rotten eggs

Identifying Minerals • Halite is the mineral form of sodium chloride (salt), so it tastes salty. • It is usually colorless or white, but impurities can make it light blue, dark blue, purple, pink, red, orange, yellow, or gray

Mineral Lab • • • Listen carefully to all directions! Pick up a mineral bag and a materials bag Streak plate = white plate The numbers DO NOT matter You MUST have 3 -4 people in your group When you are finished, put the minerals back in the minerals and materials back in their respective bags


- Slides: 39