Bellringer 1 What is an organic molecule 2

Bellringer: 1. What is an organic molecule? 2. Which of these are organic molecules? Nucleic Acids, Water, Protein, Sugar, Carbohydrates, Salt, Vitamin C. copyright cmassengale 1

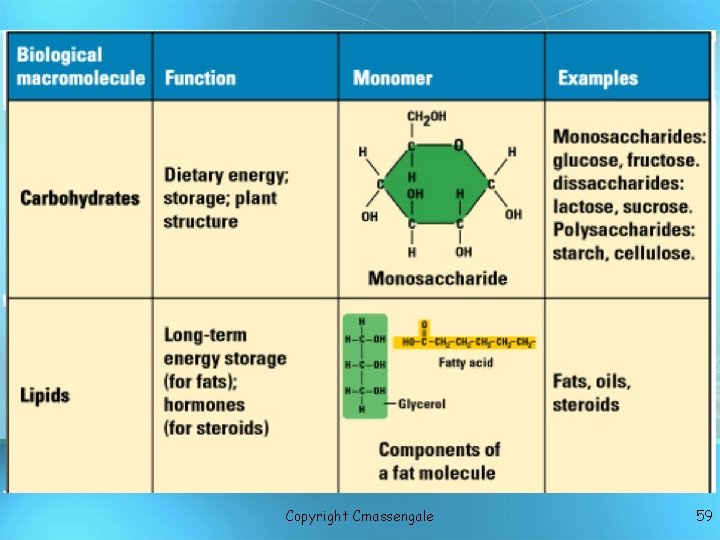
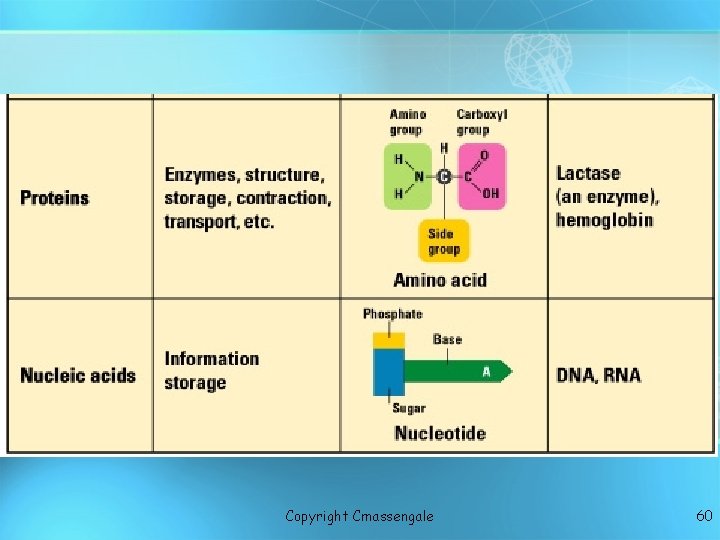
Macromolecules Proteins Nucleic acids Lipids Carbohydrates copyright cmassengale 2
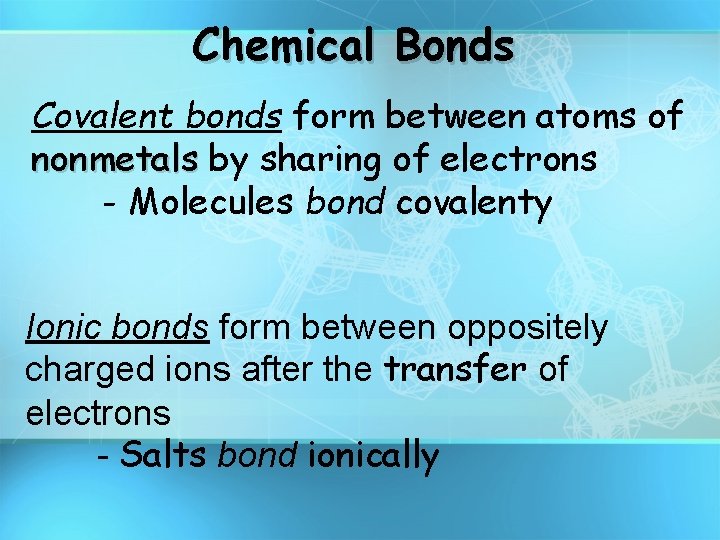
Chemical Bonds Covalent bonds form between atoms of nonmetals by sharing of electrons - Molecules bond covalenty Ionic bonds form between oppositely charged ions after the transfer of electrons - Salts bond ionically
Carbon-based Molecules • Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules • Organic chemistry is the study of carbon compounds Copyright Cmassengale 4
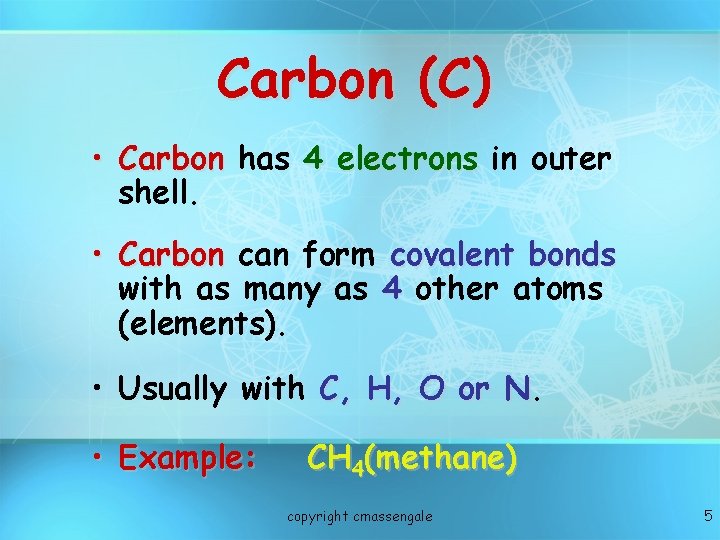
Carbon (C) • Carbon has 4 electrons in outer shell. • Carbon can form covalent bonds with as many as 4 other atoms (elements). • Usually with C, H, O or N. N • Example: CH 4(methane) copyright cmassengale 5

Organic Compounds • Compounds that contain CARBON are called organic • Macromolecules are large organic molecules copyright cmassengale 6
• • Macromolecules Large organic molecules. Also called POLYMERS Poly = many, mer = part Made up of smaller “building blocks” called MONOMERS • Examples: 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic acids (DNA and RNA) copyright cmassengale 7

Question: How Are Macromolecules Formed? copyright cmassengale 8
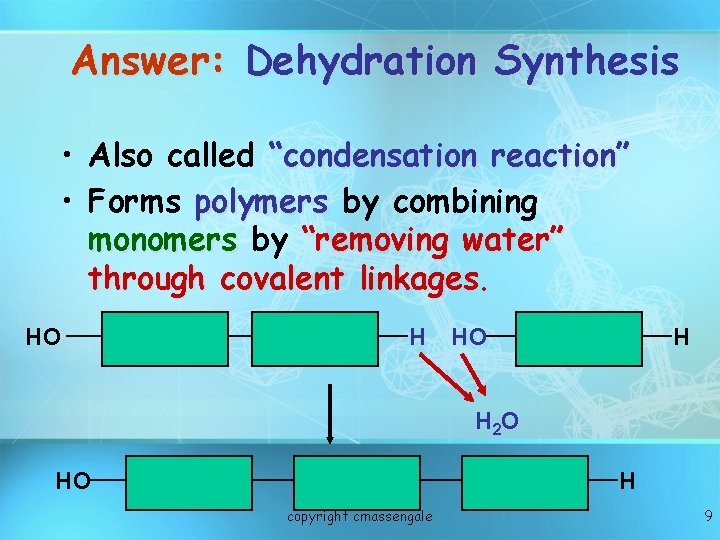
Answer: Dehydration Synthesis • Also called “condensation reaction” • Forms polymers by combining monomers by “removing water” through covalent linkages. HO H H 2 O HO H copyright cmassengale 9

Question: How are Macromolecules separated or digested? copyright cmassengale 10
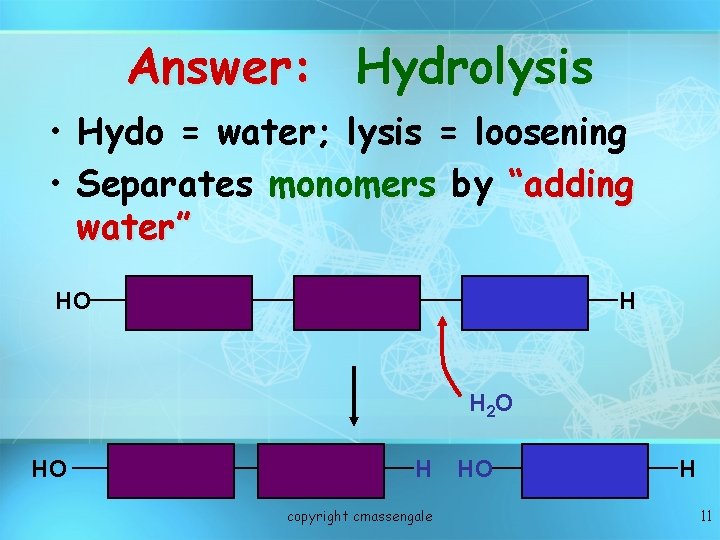
Answer: Hydrolysis • Hydo = water; lysis = loosening • Separates monomers by “adding water” HO H H 2 O HO H copyright cmassengale HO H 11
Biochemistry • Organic compounds, in which carbon atoms are combined with hydrogen and usually oxygen, are needed for life to exist. • Carbon atoms can combine in long chains that form the backbone of large complex molecules, or macromolecules.
II. Classes of Organic Molecules: • What are the four classes of organic molecules? • Carbohydrates • Lipids • Proteins • Nucleic Acids

A sucrose molecule (above) depicted as a stick molecule. Carbohydrates Glycogen is abundant in metabolically active tissues such as liver (left) and skeletal muscle (right). The glycogen stains dark magenta. copyright cmassengale Juniper sap Milk (right) contains the disaccharide, lactose. 14

A. Carbohydrates • Sugars • Carbo = carbon, hydrate = water; carbohydrates have the molecular formula (CH 2 O)n • All known types of living cells contain carbohydrates which contain carbon atoms and hydrogen and oxygen atoms in the same two-toone ratio as water.

Carbohydrates are important as both energy storage molecules and as the structural elements in cells and tissues. Sugars (mono-, di-, and trisaccharides) play a central role in energy storage. Functions: – – Store energy in chemical bonds Glucose is the most common monosaccharide Glucose is produced by photosynthetic autotrophs Structures Carbohydrates are the major component of most plants (60 -90% of dry weight). Weaving cloth Carbohydrates are used by humans as a cheap food source. . . Collecting thatch for roofing Carrying wood . . . and as a source of fuel, . . . housing and clothing. Cotton, linen, and coir are all made up of cellulose, a carbohydrate polymer.
Carbohydrates • Small sugar molecules to large sugar molecules • Three types A. monosaccharide B. disaccharide C. polysaccharide copyright cmassengale 17
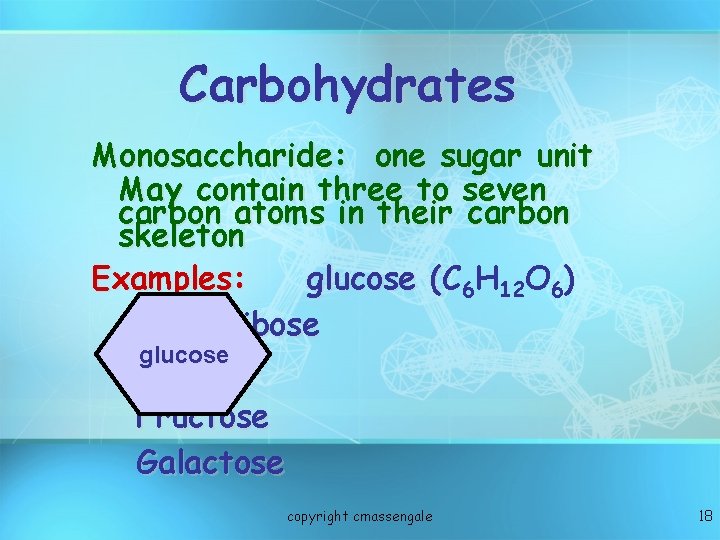
Carbohydrates Monosaccharide: one sugar unit May contain three to seven carbon atoms in their carbon skeleton Examples: glucose (C ( 6 H 12 O 6) deoxyribose glucose ribose Fructose Galactose copyright cmassengale 18
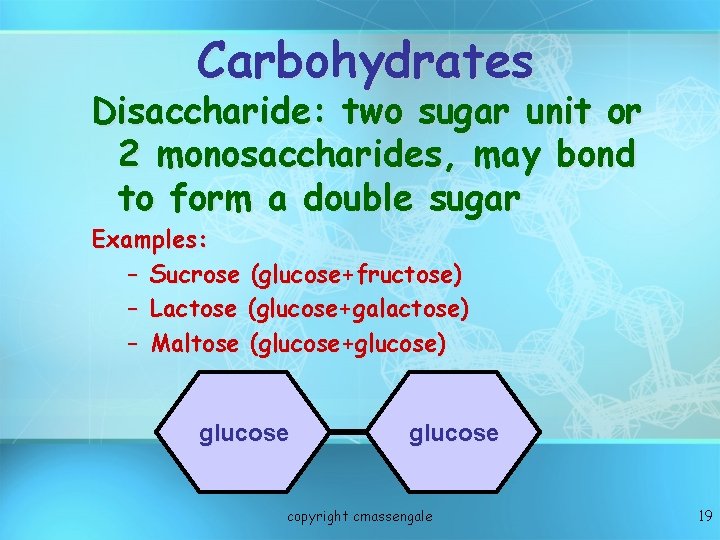
Carbohydrates Disaccharide: two sugar unit or 2 monosaccharides, may bond to form a double sugar Examples: – Sucrose (glucose+fructose) – Lactose (glucose+galactose) – Maltose (glucose+glucose) glucose copyright cmassengale 19
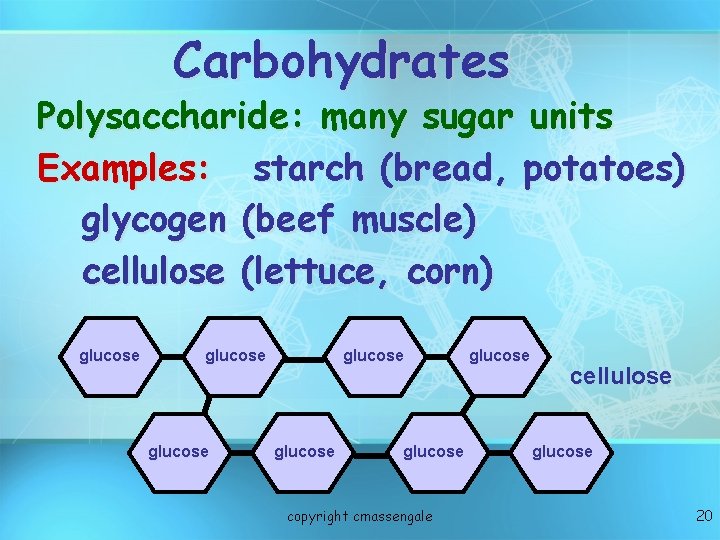
Carbohydrates Polysaccharide: many sugar units Examples: starch (bread, potatoes) glycogen (beef muscle) cellulose (lettuce, corn) glucose glucose copyright cmassengale glucose cellulose glucose 20
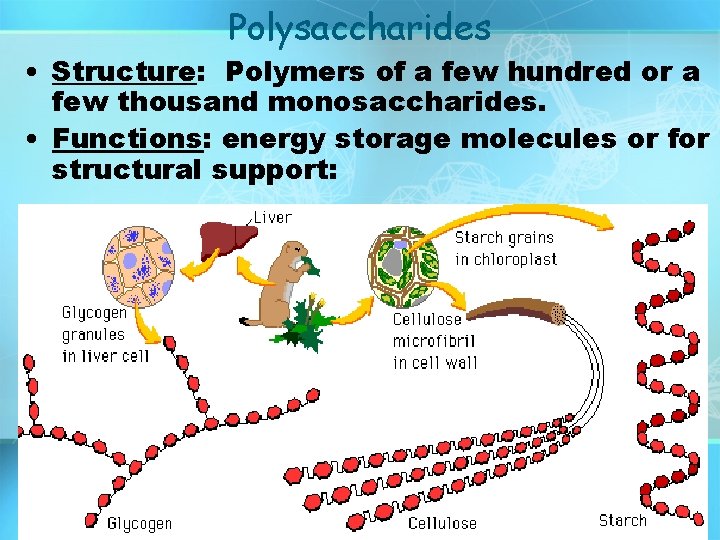
Polysaccharides • Structure: Polymers of a few hundred or a few thousand monosaccharides. • Functions: energy storage molecules or for structural support:

Mitochondrion (false color TEM) Lipids are concentrated sources of energy and can be broken down (through fatty acid oxidation in the mitochondria) to provide fuel for aerobic respiration Lipids Waxes and oils, when secreted on to surfaces provide waterproofing in plants and animals. The white fat tissue (arrows) is visible in this ox kidney Fat absorbs shocks. Organs that are prone to bumps and shocks (e. g. kidneys) are cushioned with a relatively thick layer of fat. Phospholipids form the structural framework of cellular membranes, e. g. the plasma membrane (above). copyright cmassengale Stored lipids provide insulation in extreme environments. Increased body fat levels in winter reduce heat losses to the environment. 22

Lipids • General term for compounds which are not soluble in water = nonpolar • Lipids are soluble in hydrophobic solvents • Remember: “stores the most energy” • Examples: 1. Fats/oils/triglycerides 2. Phospholipids 3. Waxes 4. Steroid hormones copyright cmassengale 23

Lipids Six functions of lipids: 1. Long term energy storage 2. Protection against heat loss (insulation) 3. Protection against physical shock 4. Protection against water loss 5. Chemical messengers (hormones) 6. Major component of membranes (phospholipids) copyright cmassengale 24
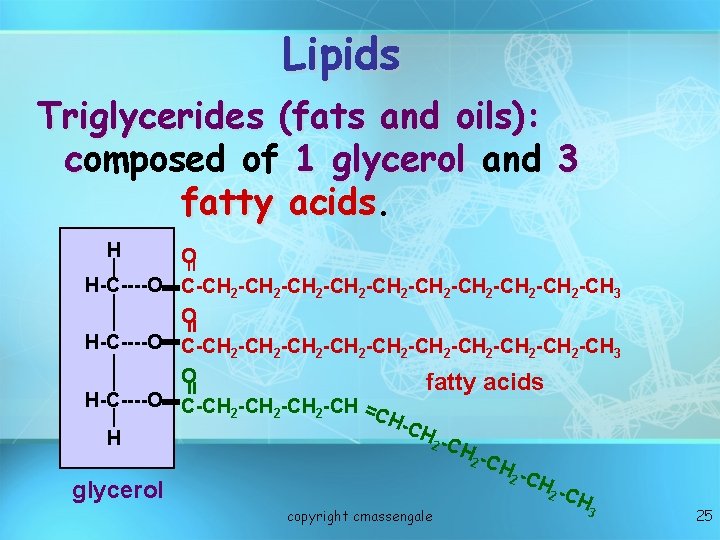
Lipids Triglycerides (fats and oils): composed of 1 glycerol and 3 fatty acids H = O H-C----O C-CH 2 -CH 2 -CH 2 -CH 2 -CH 2 -CH 3 O fatty acids H-C----O C-CH -CH = 2 2 2 CH -CH H 2 -C H 2 C Hglycerol 2 C H = copyright cmassengale 3 25

Lipid: Fatty Acids • Long chains of mostly carbon and hydrogen atoms with a -COOH group at one end. • Building blocks of lipids, called fatty acids and glycerol, make up the simple fats.

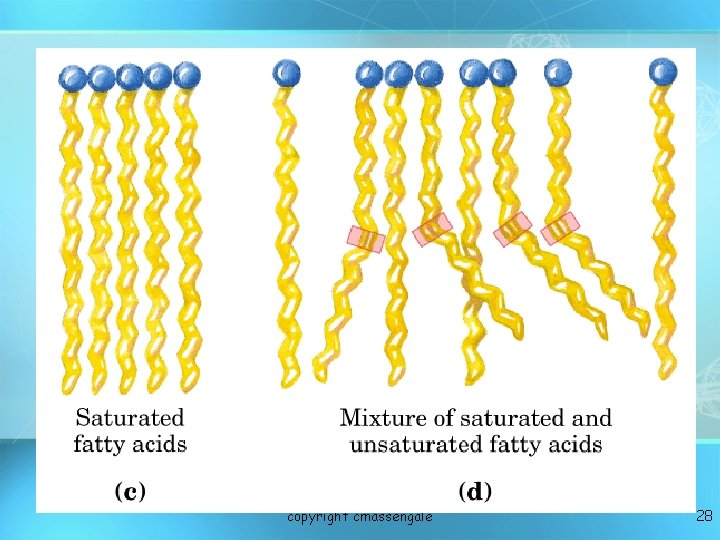
Fatty Acids Fatty acids – long hydrocarbon chains = 1. Saturated fatty acids: no double bonds (bad); solid O at room temp; most animal fats saturated C-CH 2 -CH 2 -CH 2 -CH 3 = 2. Unsaturated fatty acids: double bonds (good); liquid Oat room temp; most plant fats unsaturated C-CH 2 -CH =CH -CH 2 -C H 2 C H 3 Oils have at least one unsaturated fatty acid Fats have three saturated fatty acids 27
copyright cmassengale 28
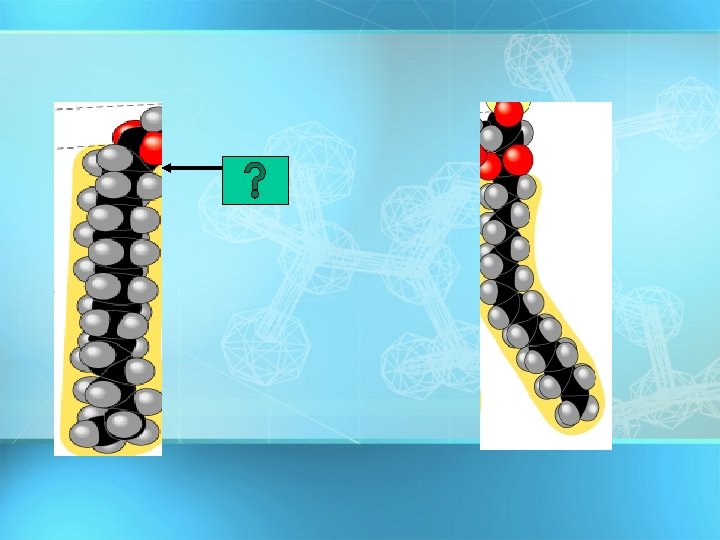
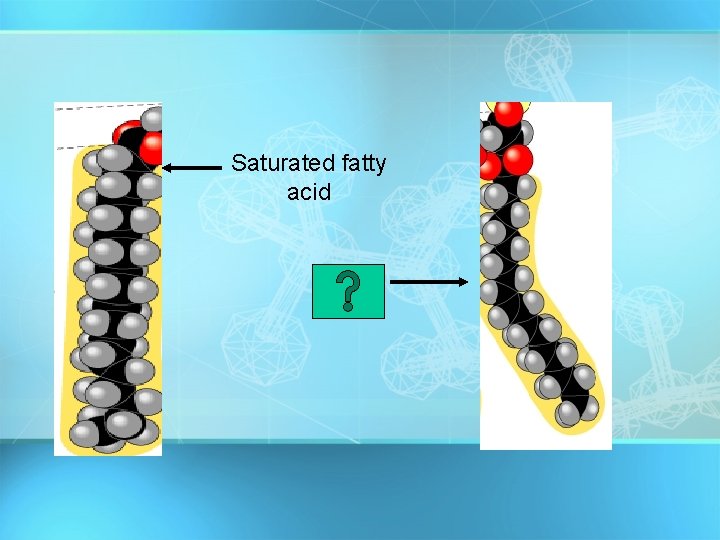
Saturated fatty acid
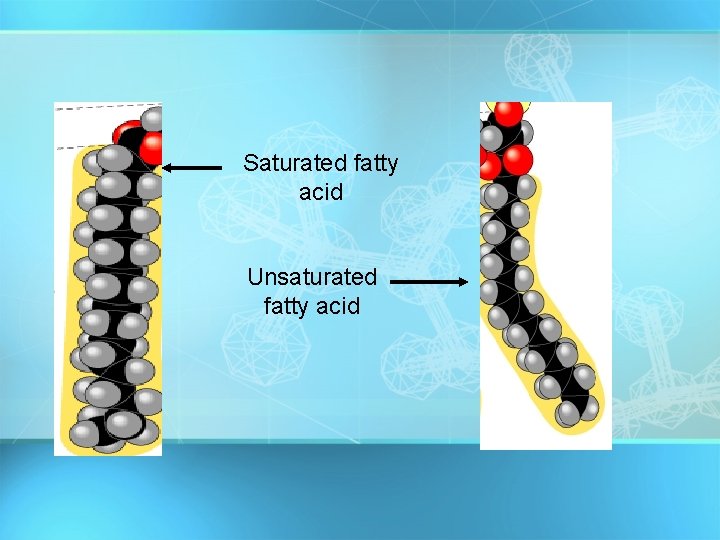
Saturated fatty acid Unsaturated fatty acid

Lipids • Fats are a more efficient form of energy storage than are carbohydrates because fats contain a larger number of hydrogen atoms and less oxygen. Two other types of lipids important in cells are phospholipids and cholesterol. Phospholipids form when a molecule of glycerol combines with two fatty acids and a phosphate group. Together with proteins, phospholipids form cellular membranes.
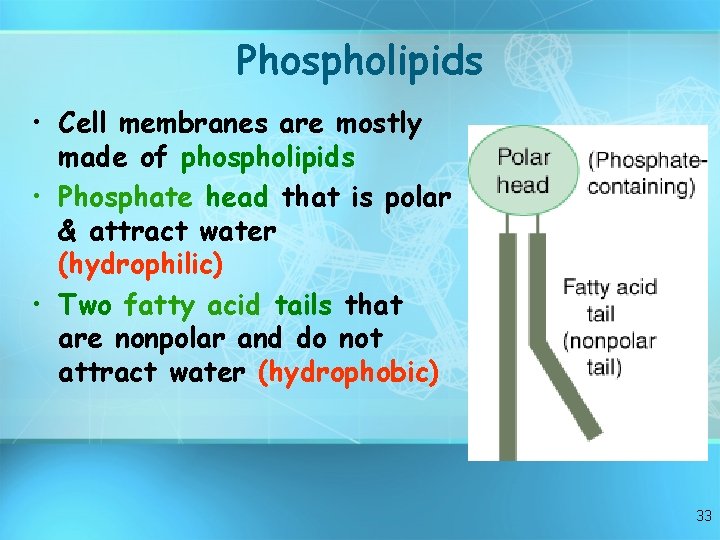
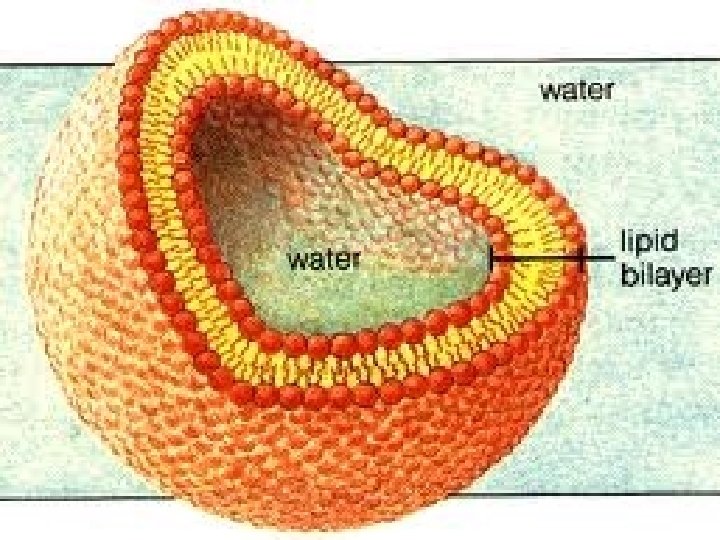
Phospholipids • Cell membranes are mostly made of phospholipids • Phosphate head that is polar & attract water (hydrophilic) • Two fatty acid tails that are nonpolar and do not attract water (hydrophobic) 33
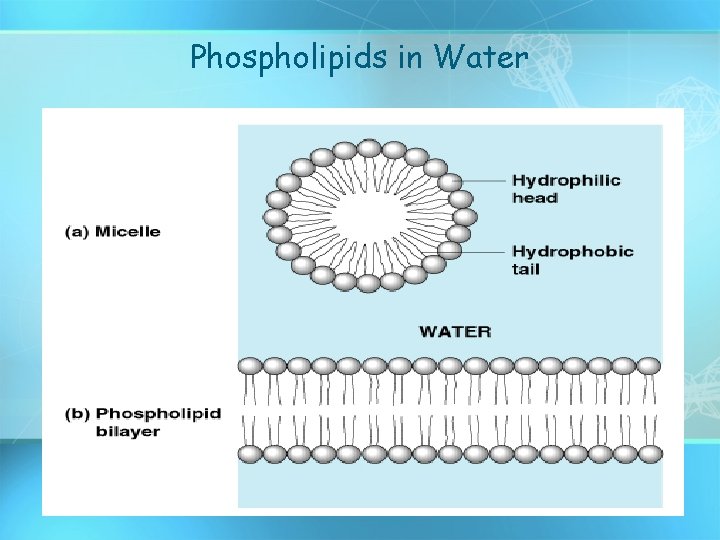
Phospholipids in Water
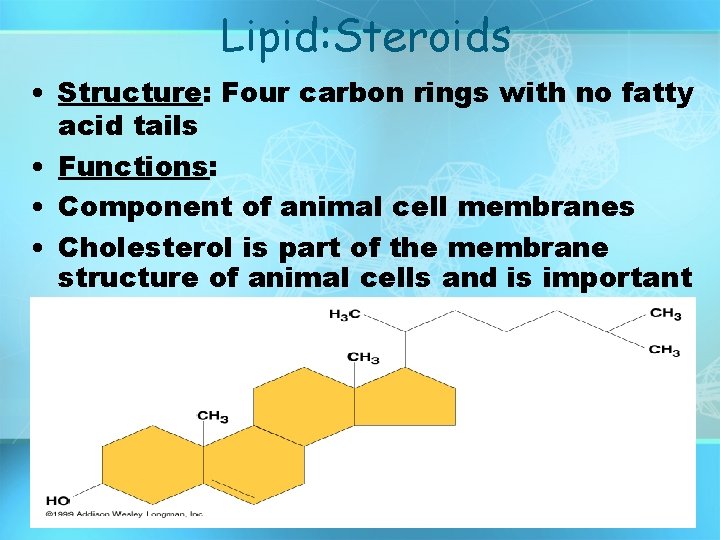
Lipid: Steroids • Structure: Four carbon rings with no fatty acid tails • Functions: • Component of animal cell membranes • Cholesterol is part of the membrane structure of animal cells and is important in nutrition.
• C_ _ _ Warm-Up: Use your • l. Actose notes to fill in the poem… • _ _ _R _ _ • Beefmuscle • O______ • H_______ • __Y_____ • Deoxyribose • R_____ • _A____ • Fruc. Tose • E_____ 37 • S_____
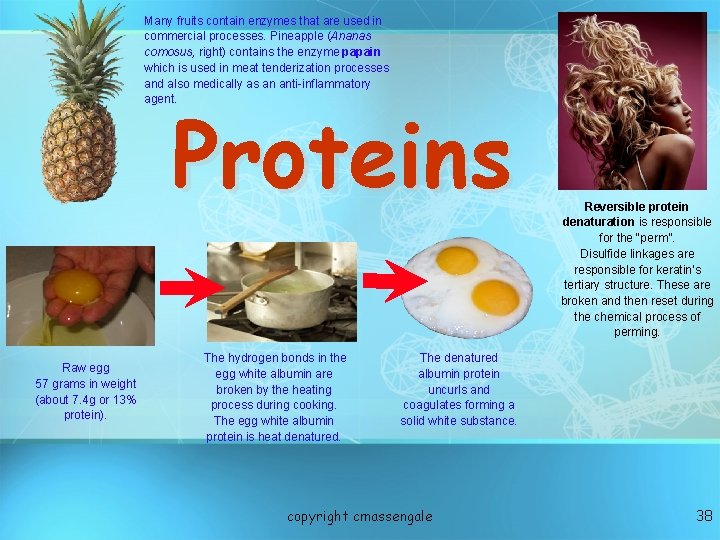
Many fruits contain enzymes that are used in commercial processes. Pineapple (Ananas comosus, right) contains the enzyme papain which is used in meat tenderization processes and also medically as an anti-inflammatory agent. Proteins Raw egg 57 grams in weight (about 7. 4 g or 13% protein). The hydrogen bonds in the egg white albumin are broken by the heating process during cooking. The egg white albumin protein is heat denatured. Reversible protein denaturation is responsible for the “perm”. Disulfide linkages are responsible for keratin’s tertiary structure. These are broken and then reset during the chemical process of perming. The denatured albumin protein uncurls and coagulates forming a solid white substance. copyright cmassengale 38
C. Proteins • Every living cell contains from several hundred to several thousand different macromolecules known as • The most essential role of proteins is as enzymes, specialized molecules that assist the many reactions occurring in cells.
Proteins (Polypeptides) • Amino acids (20 different kinds of aa) bonded together by peptide bonds (polypeptides). polypeptides • Six functions of proteins: 1. Storage: albumin (egg white) 2. Transport: hemoglobin 3. Regulatory: hormones 4. Movement: muscles 5. Structural: membranes, hair, nails 6. Enzymes: cellular reactions copyright cmassengale 40
Proteins • Structure: • Covalent bonds between the acid group of one amino acid molecule and the amino group of another are called • Additional peptide bonds may form, resulting in a long chain of amino acids, or • Have a 3 dimensional globular shape
Proteins (Polypeptides) Four levels of protein structure: A. Primary Structure B. Secondary Structure C. Tertiary Structure D. Quaternary Structure copyright cmassengale 42
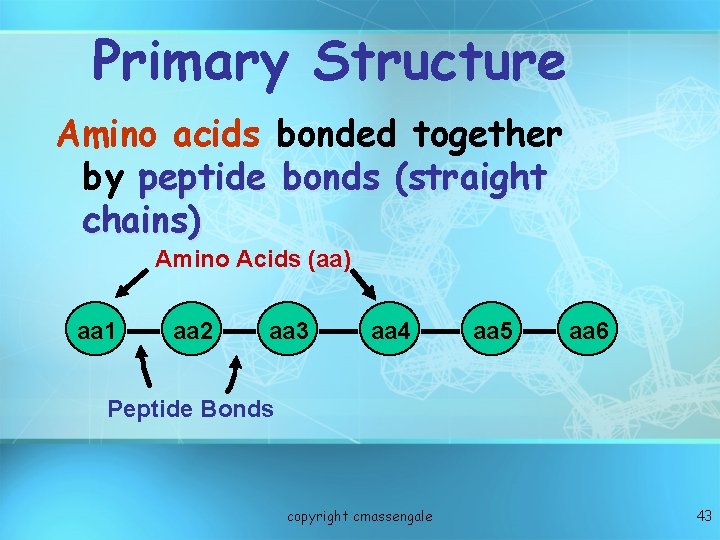
Primary Structure Amino acids bonded together by peptide bonds (straight chains) Amino Acids (aa) aa 1 aa 2 aa 3 aa 4 aa 5 aa 6 Peptide Bonds copyright cmassengale 43
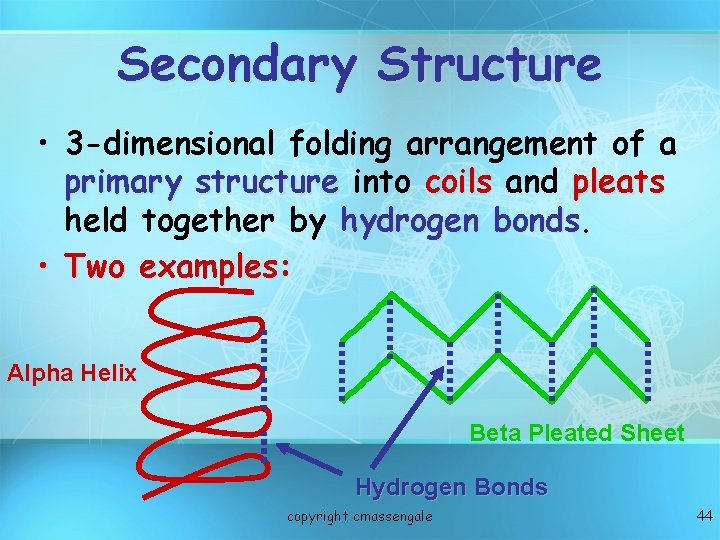
Secondary Structure • 3 -dimensional folding arrangement of a primary structure into coils and pleats held together by hydrogen bonds • Two examples: Alpha Helix Beta Pleated Sheet Hydrogen Bonds copyright cmassengale 44
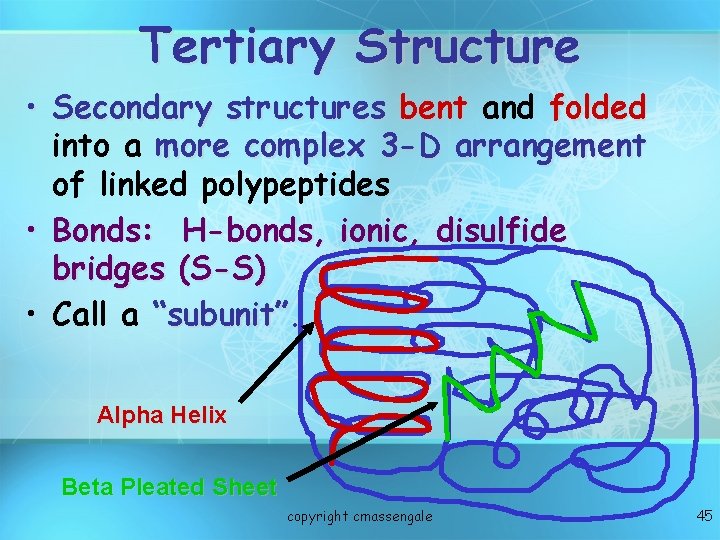
Tertiary Structure • Secondary structures bent and folded into a more complex 3 -D arrangement of linked polypeptides • Bonds: H-bonds, ionic, disulfide bridges (S-S) • Call a “subunit”. Alpha Helix Beta Pleated Sheet copyright cmassengale 45
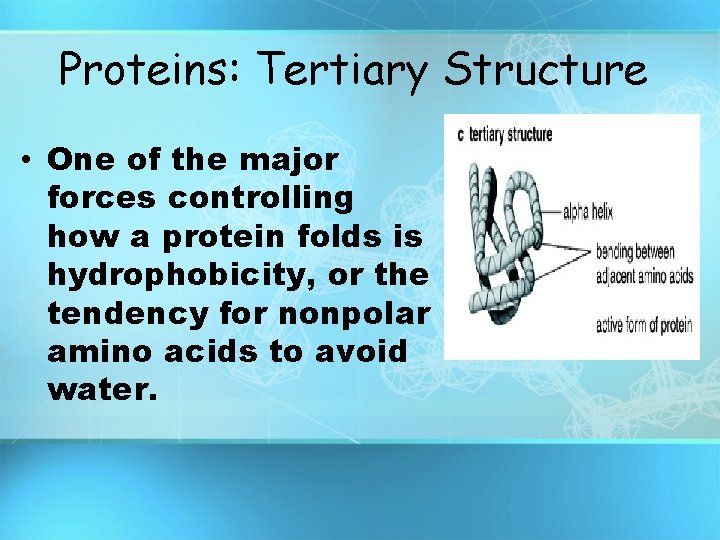
Proteins: Tertiary Structure • One of the major forces controlling how a protein folds is hydrophobicity, or the tendency for nonpolar amino acids to avoid water.
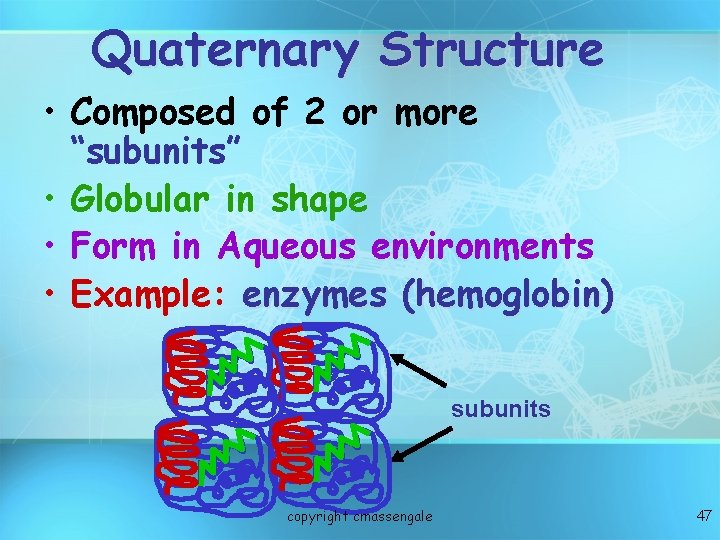
Quaternary Structure • Composed of 2 or more “subunits” • Globular in shape • Form in Aqueous environments • Example: enzymes (hemoglobin) subunits copyright cmassengale 47
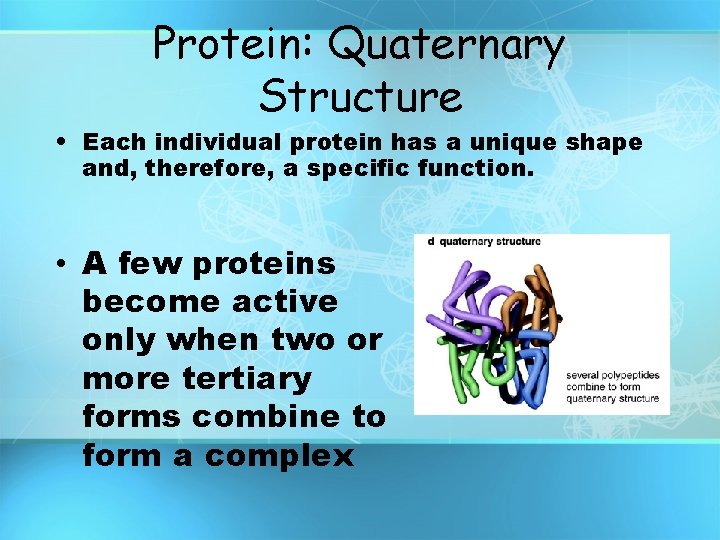
Protein: Quaternary Structure • Each individual protein has a unique shape and, therefore, a specific function. • A few proteins become active only when two or more tertiary forms combine to form a complex
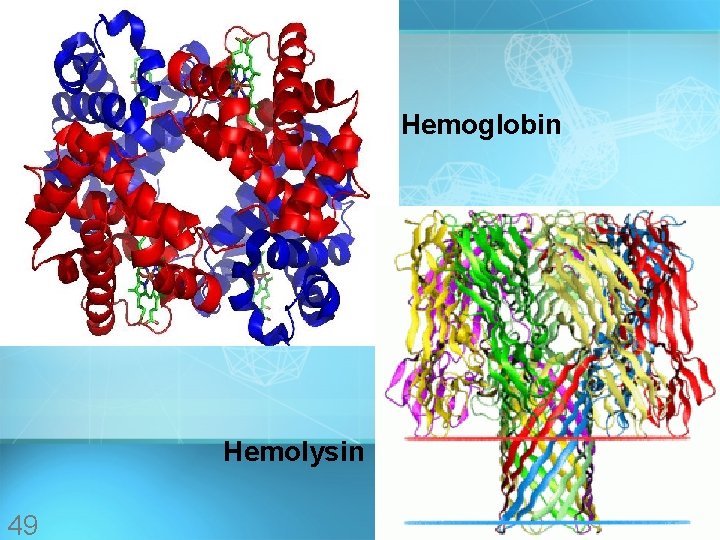
Hemoglobin Hemolysin 49
Nucleic Acids copyright cmassengale 50
Nucleic acids • Made of 5 elements: Carbon, Hydrogen, Oxygen, Nitrogen, Phosphorous (CHONP) • Two types: a. Deoxyribonucleic acid (DNAdouble helix) b. Ribonucleic acid (RNA-single strand) • Nucleic acids are composed of long chains of nucleotides linked by dehydration synthesis • Stores genetic copyright information cmassengale 51
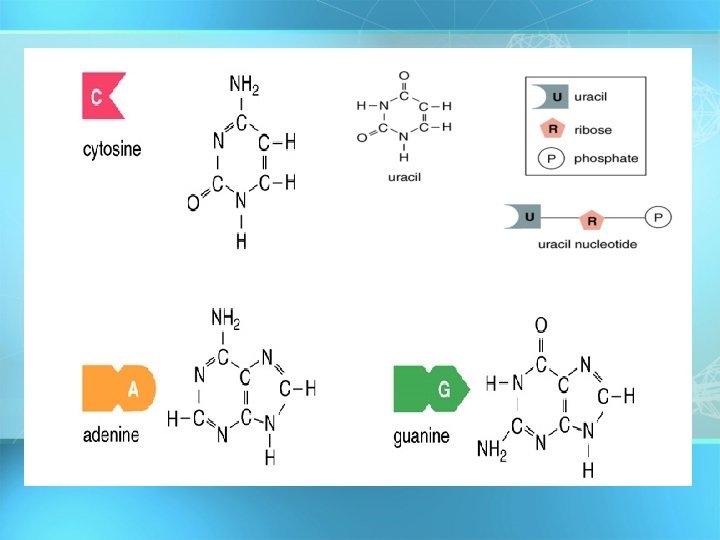
Nucleic acids • Nucleotides include: phosphate group pentose sugar (5 -carbon) nitrogenous bases: adenine (A) thymine (T) DNA only uracil (U) RNA only cytosine (C) guanine (G) copyright cmassengale 52
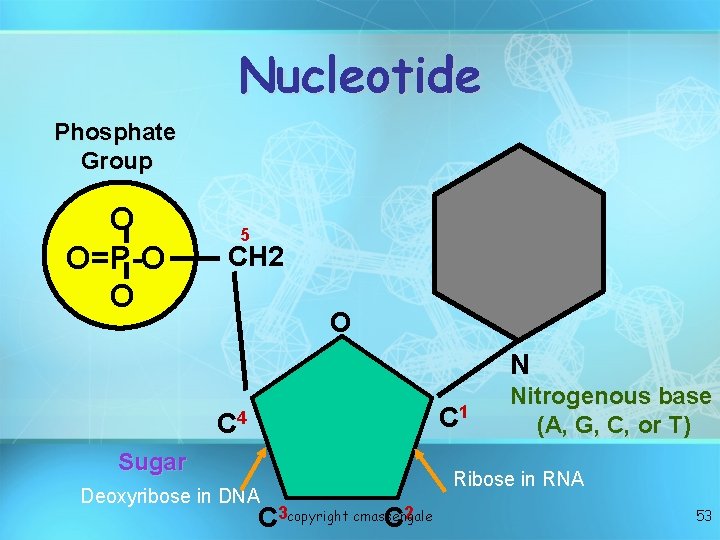
Nucleotide Phosphate Group O O=P-O O 5 CH 2 O N C 1 C 4 Sugar Deoxyribose in DNA C 3 copyright cmassengale C 2 Nitrogenous base (A, G, C, or T) Ribose in RNA 53
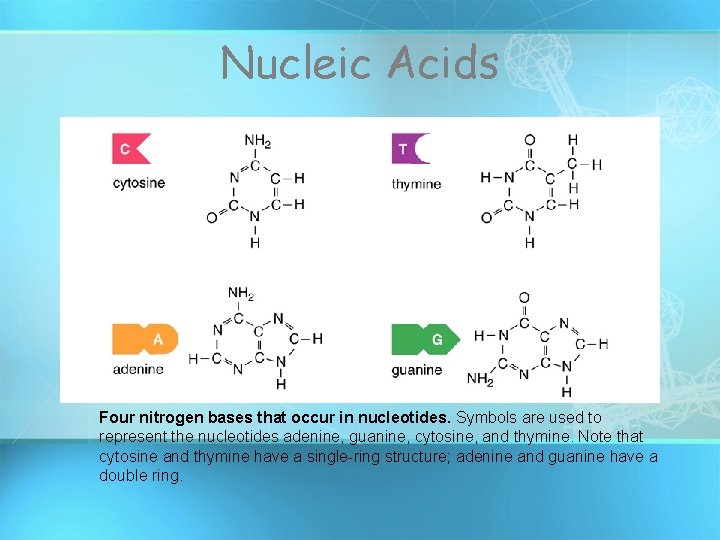
Nucleic Acids Four nitrogen bases that occur in nucleotides. Symbols are used to represent the nucleotides adenine, guanine, cytosine, and thymine. Note that cytosine and thymine have a single-ring structure; adenine and guanine have a double ring.
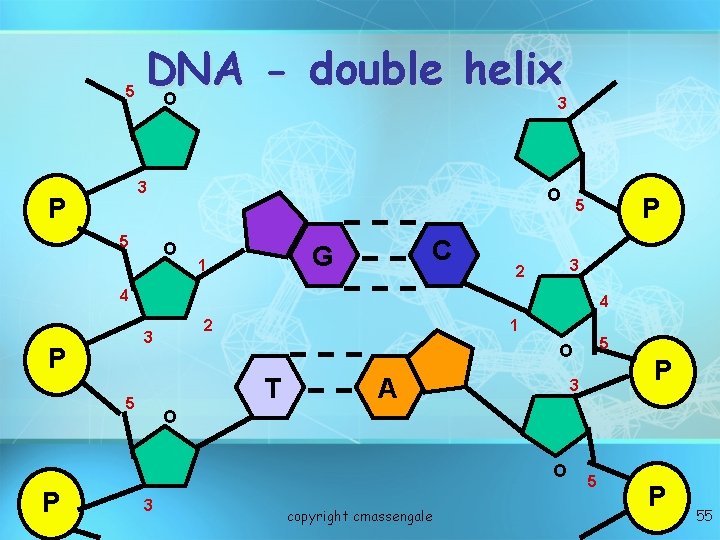
5 DNA double helix O 3 3 P 5 O O C G 1 P 5 3 2 4 4 2 3 P 1 T 5 A P 3 O O P 5 O 3 copyright cmassengale 5 P 55
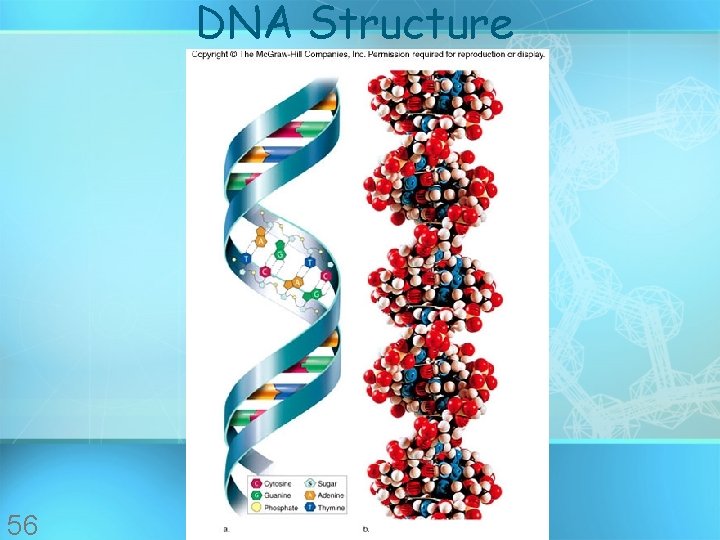
DNA Structure 56
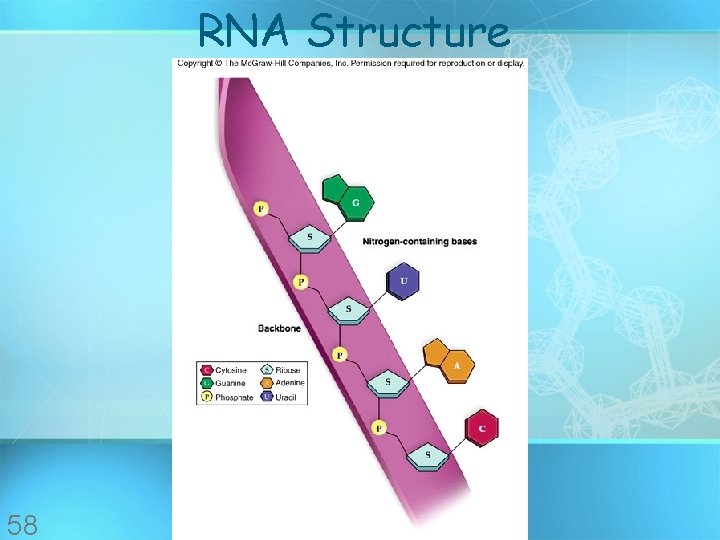
RNA Structure 58
Copyright Cmassengale 59
Copyright Cmassengale 60
copyright cmassengale 61
- Slides: 61