Bell Work What is the difference between diffusion

Bell Work What is the difference between diffusion and osmosis?

Objective Students will be able to describe the movements of substances into and out of cells. Why is it important? Understanding the mechanisms of transport across the membrane is important for understanding aspects of physiology.
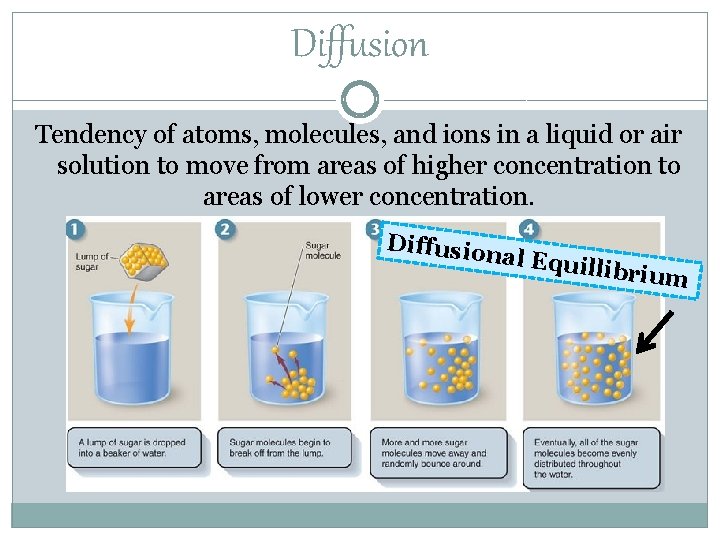
Diffusion Tendency of atoms, molecules, and ions in a liquid or air solution to move from areas of higher concentration to areas of lower concentration. Diffusio nal Equ illibrium
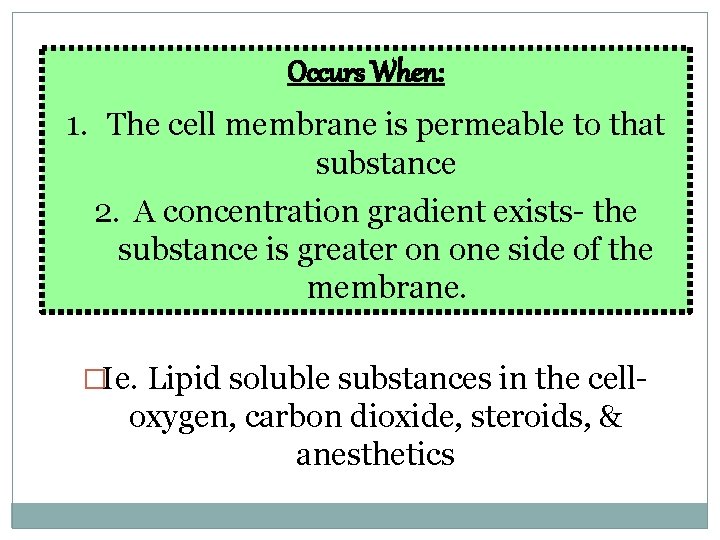
Occurs When: 1. The cell membrane is permeable to that substance 2. A concentration gradient exists- the substance is greater on one side of the membrane. �Ie. Lipid soluble substances in the cell- oxygen, carbon dioxide, steroids, & anesthetics
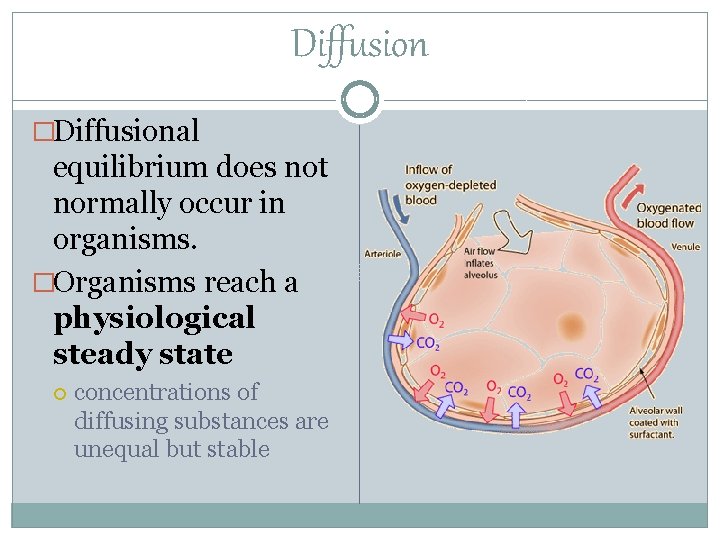
Diffusion �Diffusional equilibrium does not normally occur in organisms. �Organisms reach a physiological steady state concentrations of diffusing substances are unequal but stable
Factors of Diffusion �Rapid Diffusion occurs when there are: Shorter distances Larger concentration gradients Higher temperatures �Homeostasis maintains these factors at optimum levels.
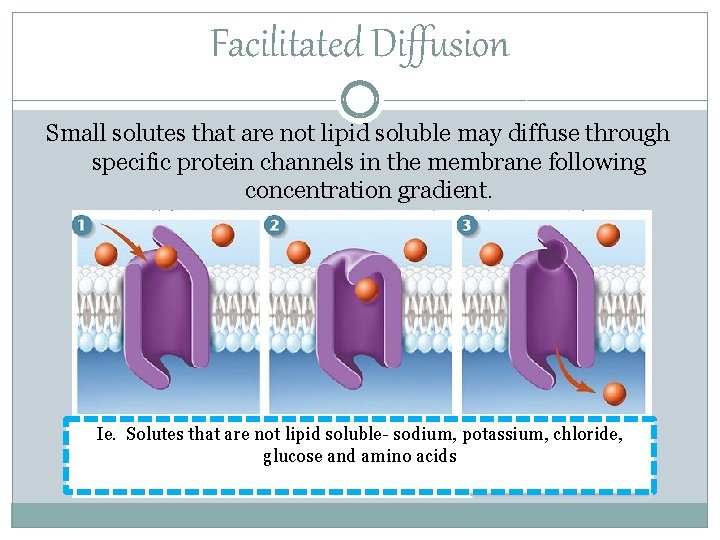
Facilitated Diffusion Small solutes that are not lipid soluble may diffuse through specific protein channels in the membrane following concentration gradient. Ie. Solutes that are not lipid soluble- sodium, potassium, chloride, glucose and amino acids
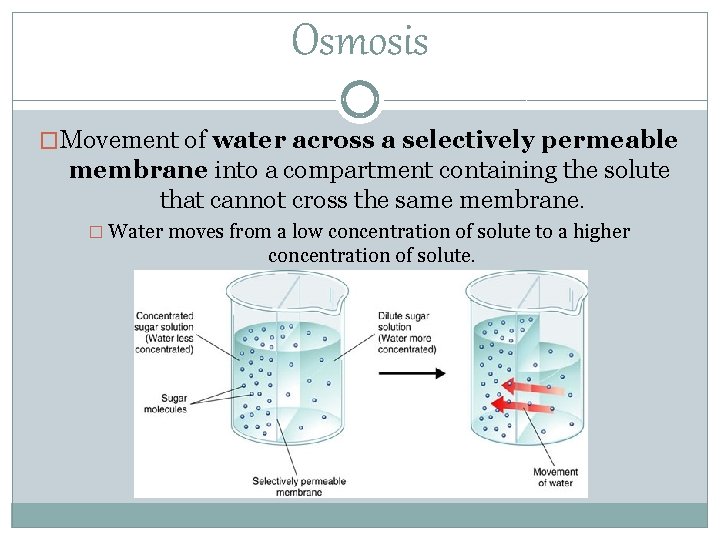
Osmosis �Movement of water across a selectively permeable membrane into a compartment containing the solute that cannot cross the same membrane. � Water moves from a low concentration of solute to a higher concentration of solute.

Cells & Osmosis �The concentration of the solution that the cell is in, will affect the state of the cell. �Three types of solutions: “Iso”tonic- think “same” “Hyper”tonic- think “above” “Hypo”tonic- think “below”
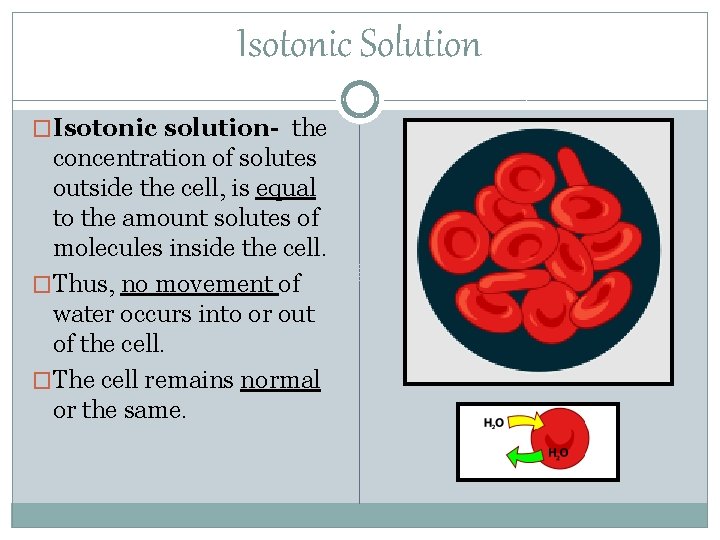
Isotonic Solution �Isotonic solution- the concentration of solutes outside the cell, is equal to the amount solutes of molecules inside the cell. �Thus, no movement of water occurs into or out of the cell. �The cell remains normal or the same.
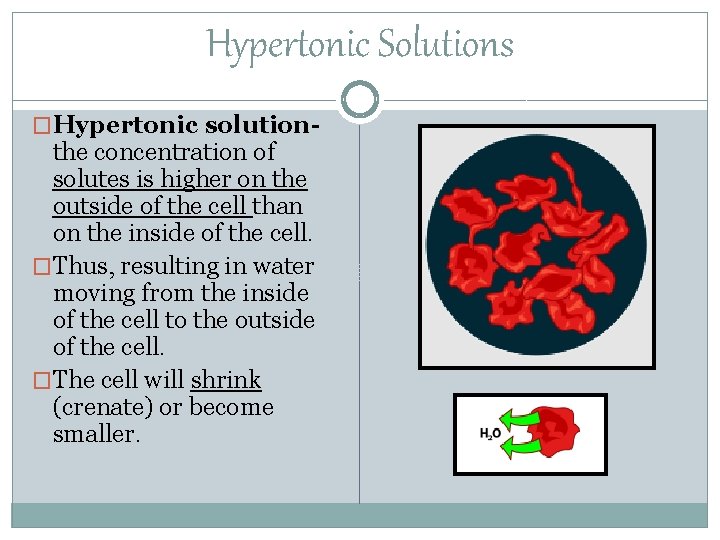
Hypertonic Solutions �Hypertonic solution- the concentration of solutes is higher on the outside of the cell than on the inside of the cell. �Thus, resulting in water moving from the inside of the cell to the outside of the cell. �The cell will shrink (crenate) or become smaller.
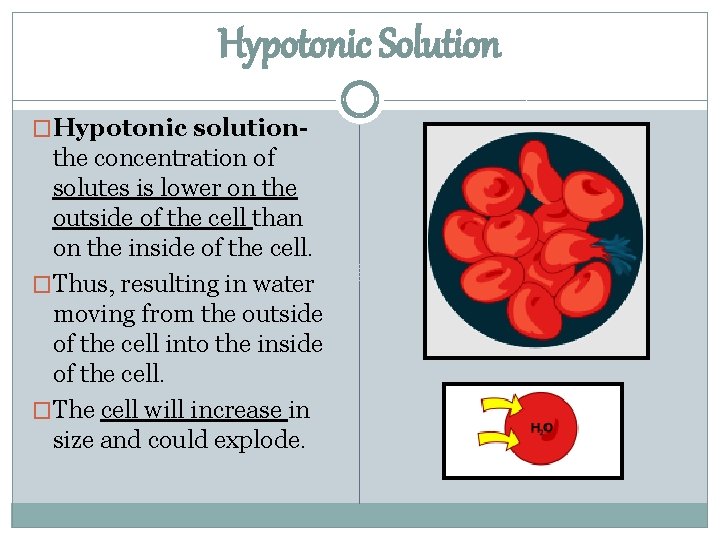
Hypotonic Solution �Hypotonic solution- the concentration of solutes is lower on the outside of the cell than on the inside of the cell. �Thus, resulting in water moving from the outside of the cell into the inside of the cell. �The cell will increase in size and could explode.
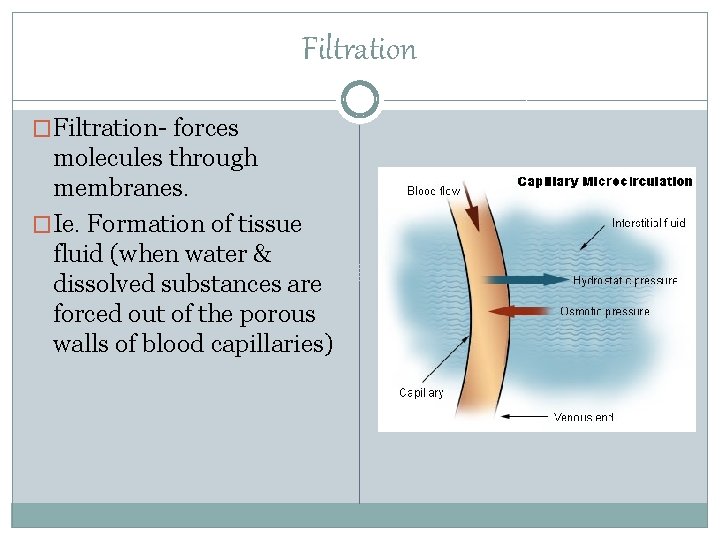
Filtration �Filtration- forces molecules through membranes. �Ie. Formation of tissue fluid (when water & dissolved substances are forced out of the porous walls of blood capillaries)
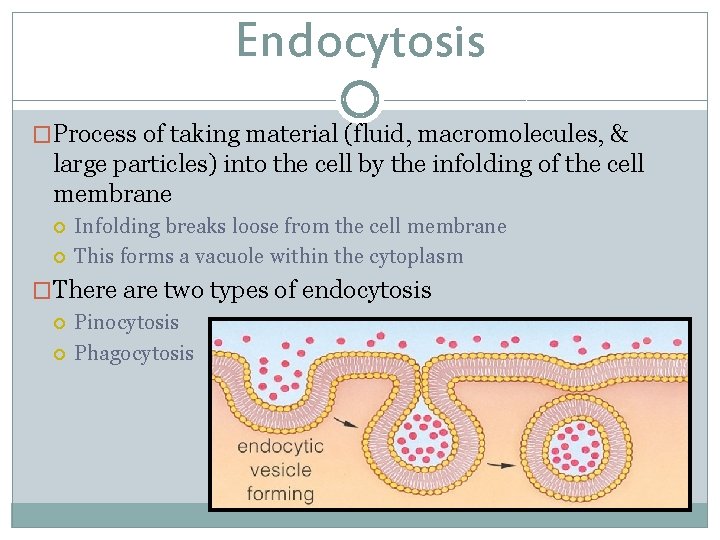
Endocytosis �Process of taking material (fluid, macromolecules, & large particles) into the cell by the infolding of the cell membrane Infolding breaks loose from the cell membrane This forms a vacuole within the cytoplasm �There are two types of endocytosis Pinocytosis Phagocytosis
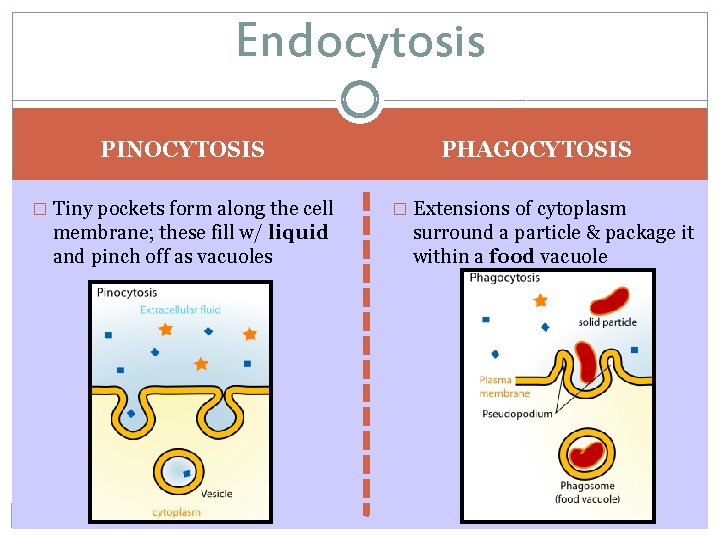
Endocytosis PINOCYTOSIS � Tiny pockets form along the cell membrane; these fill w/ liquid and pinch off as vacuoles PHAGOCYTOSIS � Extensions of cytoplasm surround a particle & package it within a food vacuole
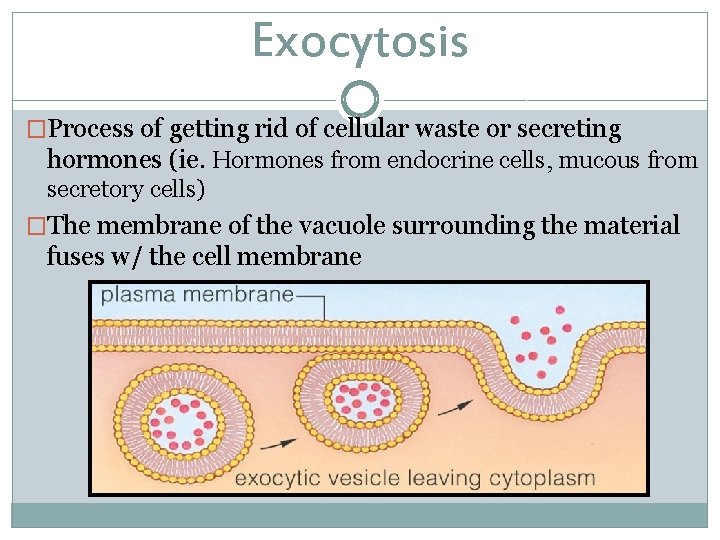
Exocytosis �Process of getting rid of cellular waste or secreting hormones (ie. Hormones from endocrine cells, mucous from secretory cells) �The membrane of the vacuole surrounding the material fuses w/ the cell membrane
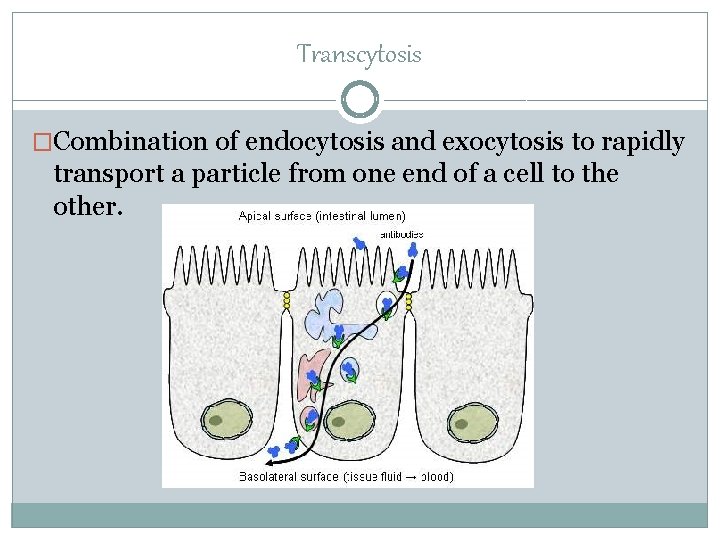
Transcytosis �Combination of endocytosis and exocytosis to rapidly transport a particle from one end of a cell to the other.
- Slides: 17