Bell Work 82119 6 Give an example of

Bell Work 8/21/19 6. Give an example of an 1. 0. 00766 hg = ____ cg irreversible physical 2. 0. 5 dm = ____ m change. 3. What should a scientific 7. What is the difference conclusion reference? between a homogeneous 4. Name 1 extensive and 1 and a heterogeneous intensive property. mixture? 5. What is the definition of a substance?

8/21/19 – Chemical properties/changes Video: https: //www. youtube. com/watch? v=JSi. BSSFKRw. E

2. 3 Distinguishing Elements and Compounds ◦ An _______ is the simplest form of matter that has a unique set of properties. ◦ A _____ is a substance that contains two or more elements chemically combined in a fixed proportion.

2. 3 Distinguishing Elements and Compounds ◦ An element is the simplest form of matter that has a unique set of properties. ◦ A compound is a substance that contains two or more elements chemically combined in a fixed proportion.

2. 3 Distinguishing Elements and Compounds How do you tell the difference between an element and a compound?

2. 3 Distinguishing Elements and Compounds How do you tell the difference between an element and a compound? Compounds can be broken down into simpler substances by chemical means, but elements cannot.

2. 3 Distinguishing Elements and Compounds ◦ Breaking Down Compounds ◦ A _______is a change that produces matter with a different composition than the original matter. ◦ Example: when table sugar is heated, it goes through a series of chemical changes.

2. 3 Distinguishing Elements and Compounds ◦ Breaking Down Compounds ◦ A chemical change is a change that produces matter with a different composition than the original matter. ◦ Example: when table sugar is heated, it goes through a series of chemical changes.

2. 3 Distinguishing Elements and Compounds ◦ In general, the properties of compounds are ____ the same as the elements that make them up. ◦ Example: Chemically combining the elements sodium and chlorine to form sodium chloride causes a change in composition and a change in properties.

2. 3 Distinguishing Elements and Compounds ◦ In general, the properties of compounds are NOT the same as the elements that make them up. ◦ Example: Chemically combining the elements sodium and chlorine to form sodium chloride causes a change in composition and a change in properties.

2. 3 Distinguishing Elements and Compounds ◦ Chlorine is an element used to kill harmful organisms in swimming pools.

2. 3 Distinguishing Elements and Compounds ◦ Sodium is a metallic element that undergoes an explosive reaction with water. ◦ https: //www. youtube. co m/watch? v=ODf_s. Pex. S 2 Q

2. 3 Distinguishing Elements and Compounds ◦ Sodium Chloride (commonly known as table salt) is a compound used to season or preserve food.
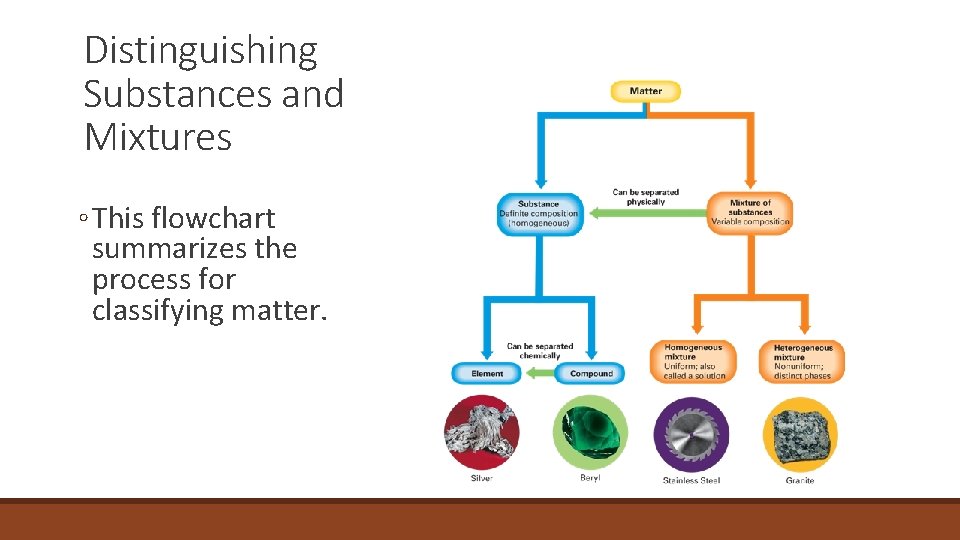
Distinguishing Substances and Mixtures ◦ This flowchart summarizes the process for classifying matter.

Example book problem
2. 4 Types of Changes ◦ During a _______, the composition of matter always changes. ◦ Remember: during a physical change, the composition of matter never changes.

2. 4 Types of Changes ◦ During a chemical change, the composition of matter always changes. ◦ Remember: during a physical change, the composition of matter never changes.

2. 4 Chemical Changes ◦ The ability of a ____ to undergo a specific chemical change is called a _______. ◦ Chemical properties can be used to identify a substance. But chemical properties can be observed only when a substance undergoes a chemical change.

2. 4 Chemical Changes ◦ The ability of a substance to undergo a specific chemical change is called a chemical property. ◦ Chemical properties can be used to identify a substance. But chemical properties can be observed only when a substance undergoes a chemical change.

2. 4 Chemical Changes ◦ A magnet separating iron from sulfur is an example of a _______ change.

2. 4 Chemical Changes ◦ A magnet separating iron from sulfur is an example of a physical change.

2. 4 Chemical Changes ◦ A mixture of iron and sulfur is heated. The iron and sulfur react and form iron sulfide. This is an example of a _______ change.

2. 4 Chemical Changes ◦ A mixture of iron and sulfur is heated. The iron and sulfur react and form iron sulfide. This is an example of a chemical change.
- Slides: 23