Bell Ringer Please pull out your Periodic Table

Bell Ringer: Please pull out your Periodic Table Lets looks at the element: Chlorine 1. What is it’s Element Symbol? 2. What is it’s Atomic #? 3. How many Protons? 4. How many Neutrons? 5. How many Electrons? 6. Draw a Bohr model of Chlorine 7. How many electrons are in the valence shell? 8. Is the element stable or unstable?
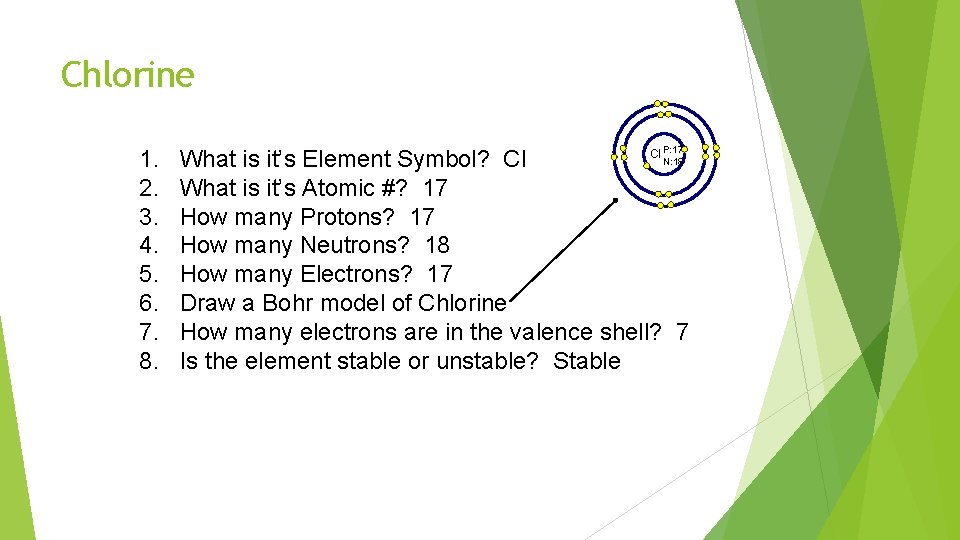
Chlorine 1. 2. 3. 4. 5. 6. 7. 8. P: 17 Cl N: 18 What is it’s Element Symbol? Cl What is it’s Atomic #? 17 How many Protons? 17 How many Neutrons? 18 How many Electrons? 17 Draw a Bohr model of Chlorine How many electrons are in the valence shell? 7 Is the element stable or unstable? Stable

What is the. Springfield High School Mascot on the Simpsons?

Isotopes: Atoms of the same element that differ in the number of neutrons that they contain. Radioactive Isotopes: Isotopes are unstable and break down at a constant rate over time. Dangerous but has practical uses. Dating rock layers, cancer treatment, food preservation.
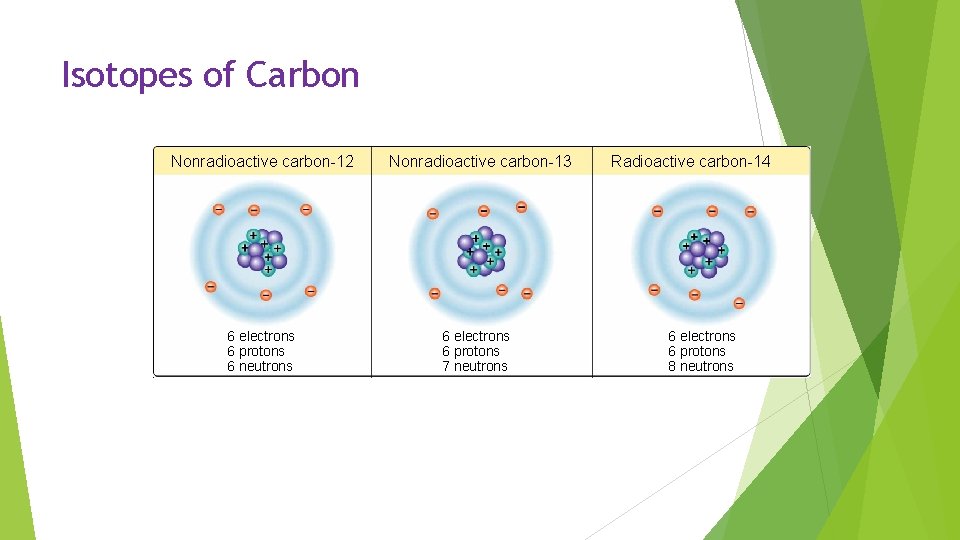
Isotopes of Carbon Nonradioactive carbon-12 6 electrons 6 protons 6 neutrons Nonradioactive carbon-13 6 electrons 6 protons 7 neutrons Radioactive carbon-14 6 electrons 6 protons 8 neutrons

Chemical Compounds Compound: A combination of two or more elements. Molecule: Written the smallest unit of a compound. as a chemical formula. Water is written as H 2 O because it contains two atoms of Hydrogen and one atom of Oxygen. Table Salt is written as Na. Cl because it has one atom of Sodium and one atom of Chlorine.
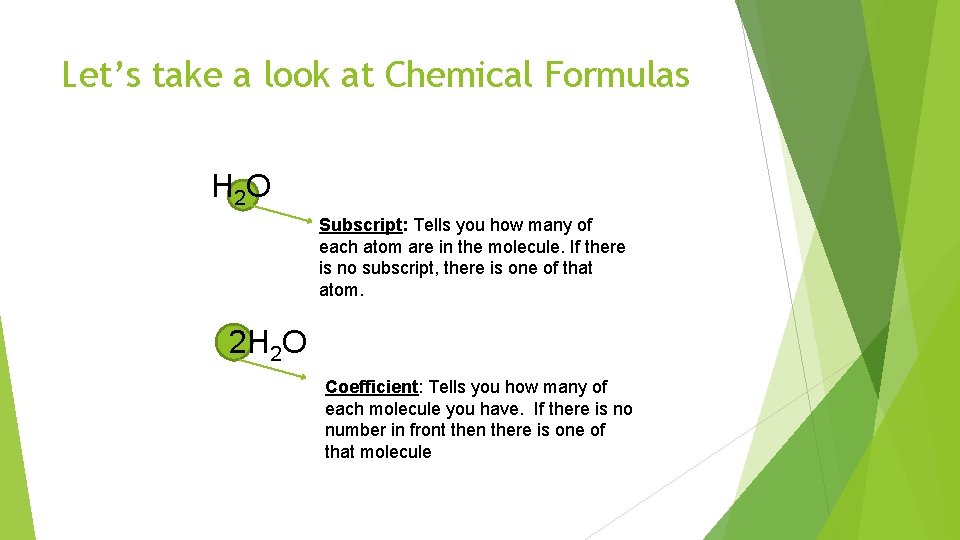
Let’s take a look at Chemical Formulas H 2 O Subscript: Tells you how many of each atom are in the molecule. If there is no subscript, there is one of that atom. 2 H 2 O Coefficient: Tells you how many of each molecule you have. If there is no number in front then there is one of that molecule

Count the Atoms! Ø H 2 O Ø 2 H 2 O Ø H 2(SO 4) Ø C 7 H 5(NO 2)3 = H: 1 = H: 2 O: 1 ***Molecules in parentheses are called Polyatomic Ions and always occur together= when they bond. H: 4 O: 2 = H: 2 S: 1 O: 4 = C: 7 H: 5 N: 3 O: 6

Chemical Bonds Ionic Bonds: Occurs when one or more electrons are transferred from one atom to another. Ion: an atom with a positive or negative charge. Ionic Bond = Giver + Taker Givers won’t bond with Givers
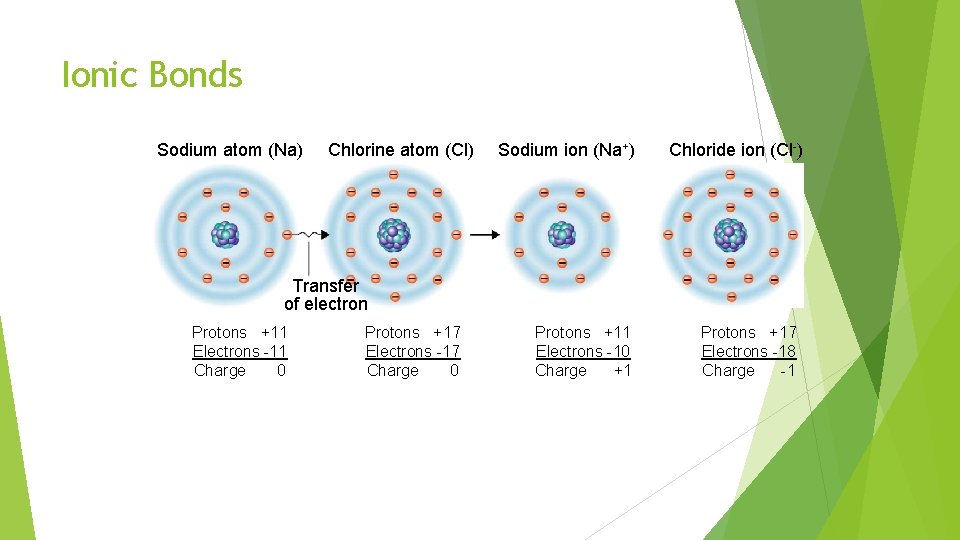
Ionic Bonds Sodium atom (Na) Chlorine atom (Cl) Sodium ion (Na+) Chloride ion (Cl-) Transfer of electron Protons +11 Electrons -11 Charge 0 Protons +17 Electrons -17 Charge 0 Protons +11 Electrons -10 Charge +1 Protons +17 Electrons -18 Charge -1

Chemical Bonds Covalent Bond: Occurs when two atoms share electrons Covalent Bond = Taker + Taker Givers won’t bond with Givers

Complete the following today Counting atoms worksheet Chemical bonding worksheet Anything you didn’t finish last class

- Slides: 13