Bell Ringer Name the oceanmapping technology Maps as

Bell Ringer • • Name the ocean-mapping technology. Maps as it orbits the Earth Topex/Poseidon Maps from a ship using sound waves SONAR Maps by diving deep in the ocean submersibles

Saltwater Properties

Ocean Water • Oceans contain 97. 5% of water found on Earth (saltwater) • What else is in ocean water? – Nutrients – Dissolved minerals – Dissolved gases – Microscopic life forms • All of these combined make the ocean a thriving ecosystem for the organisms that live there

Ocean Water • The Arctic and Antarctic Oceans are covered by sea ice

Ocean Water • Icebergs – large masses of ice formed when ice crystals build on each other

Ocean Water • As the Earth’s global temperatures continue to increase, sea ice is melting, changing the saltiness of the water as well as the sea levels • Sea Level – the average level for the surface of Earth’s oceans – Serves as a reference for elevation (ex: Raleigh is 315 feet above sea level)
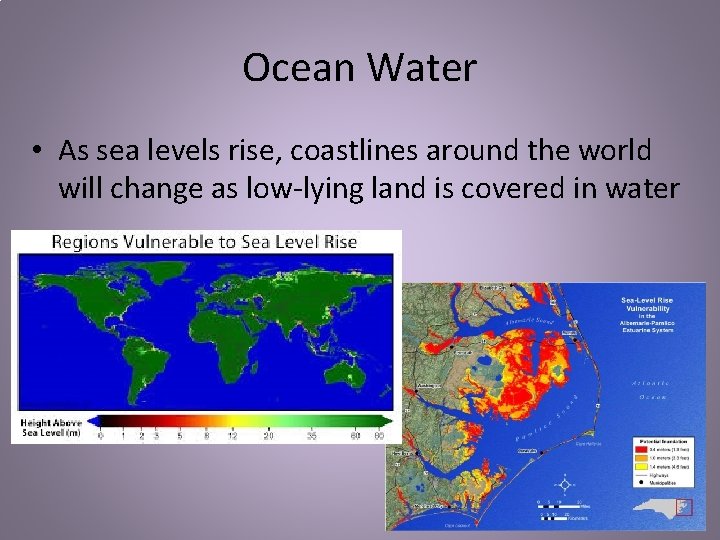
Ocean Water • As sea levels rise, coastlines around the world will change as low-lying land is covered in water

Ocean Water • Density = mass / volume • Things that are denser [rise above / sink below] things that are less dense. • Sea ice floats, so it is [more / less] dense than water Ice floats because it is less dense than water

Ocean Water • The density of ocean water depends on two things: salinity and temperature

Salinity • Seawater is a solution of about 96. 5% water and 3. 5% dissolved salts • The most abundant salt in sea water is sodium chloride (Na. Cl, table salt)

Salinity • Salinity – the amount of dissolved salts in seawater • Salt adds weight (mass) to water, so saltwater is denser than freshwater

Salinity • Adding salt to water lowers its freezing point • So the higher the salinity, the colder the water can be and still be a liquid • This is why we put salt on roads in winter to prevent them from freezing

Salinity • The average ocean salinity is 35 ppt (parts per thousand) • Salinity stays relatively constant in the ocean because sea salts are removed at the same rate they are added

Salinity • How are salts removed from ocean water? • Marine organisms remove salt from the water to build their shells, bones, and teeth • Salts can precipitate from (drop to the bottom of) seawater in dry and coastal regions
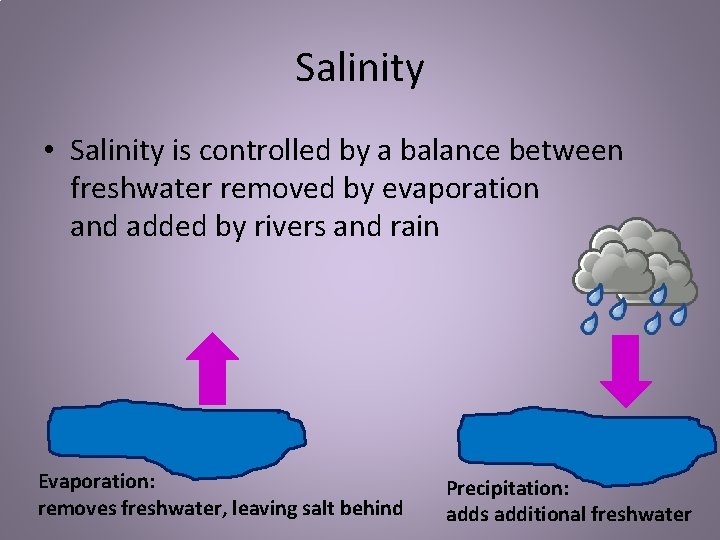
Salinity • Salinity is controlled by a balance between freshwater removed by evaporation and added by rivers and rain Evaporation: removes freshwater, leaving salt behind Precipitation: adds additional freshwater
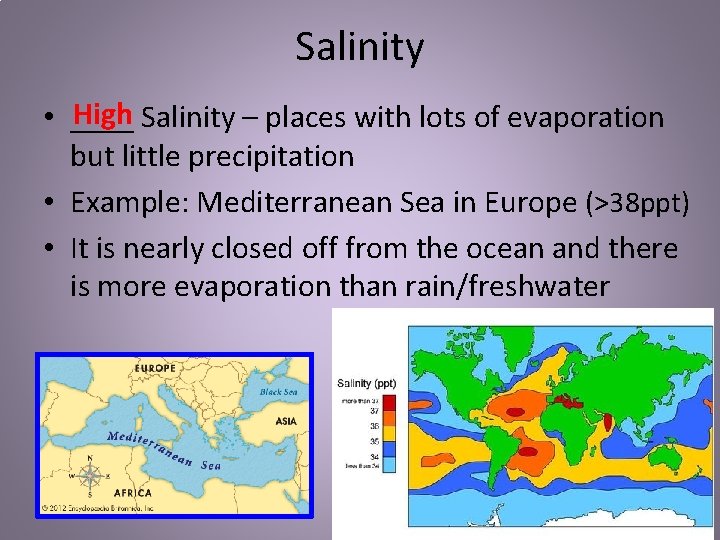
Salinity High Salinity – places with lots of evaporation • ____ but little precipitation • Example: Mediterranean Sea in Europe (>38 ppt) • It is nearly closed off from the ocean and there is more evaporation than rain/freshwater

Salinity Low Salinity – places with lots of rain that • _____ dilutes the water

Salinity Low Salinity – places where freshwater • _____ estuaries streams empty into the ocean (______) • Example: Baltic Sea in Europe (10 ppt) • It’s nearly closed off from the ocean and has hundreds of rivers that empty into it
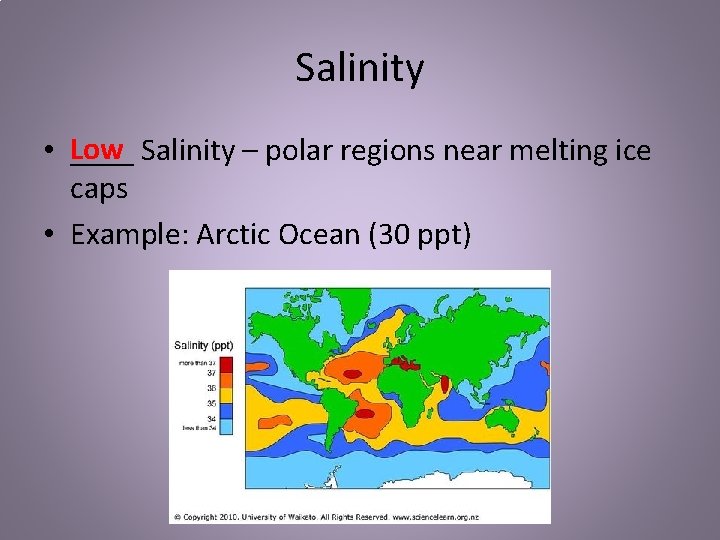
Salinity • Low ____ Salinity – polar regions near melting ice caps • Example: Arctic Ocean (30 ppt)

Temperatures • • • Average ocean temperature is 15 o. C (59 o. F) Equatorial waters are warmest, averaging 30 o. C Polar waters are coldest, averaging -2 o. C 0 o. C. Freshwater freezes at ___ So how is the water still liquid? Remember, saltwater freezes at a lower temperature than freshwater

Temperatures • Density is temperature-dependent, so ocean temperatures also vary based on depth – Cold water – dense – Warm water – less dense • Cold water sinks to the lower parts of the ocean while warm water remains near the surface rises • Review: Convection: warm material _______ sinks and cold material _______!

Temperatures • The ocean is divided into three temperature-based layers: – Surface Layer – Thermocline – Deep Water
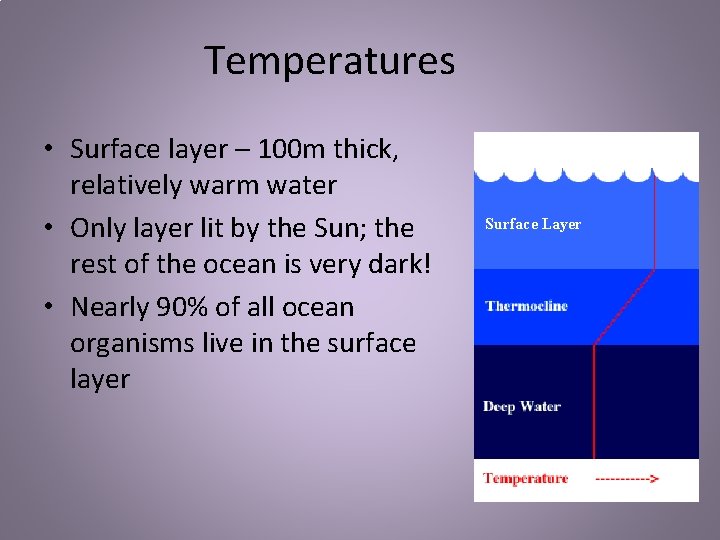
Temperatures • Surface layer – 100 m thick, relatively warm water • Only layer lit by the Sun; the rest of the ocean is very dark! • Nearly 90% of all ocean organisms live in the surface layer Surface Layer
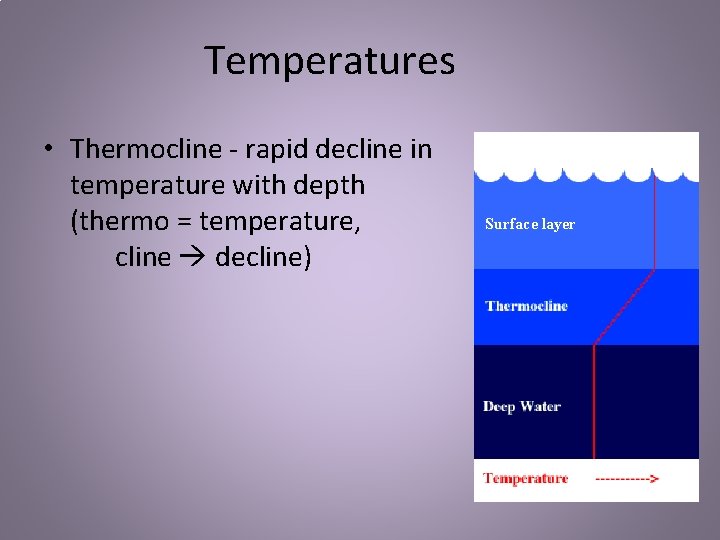
Temperatures • Thermocline - rapid decline in temperature with depth (thermo = temperature, cline decline) Surface layer
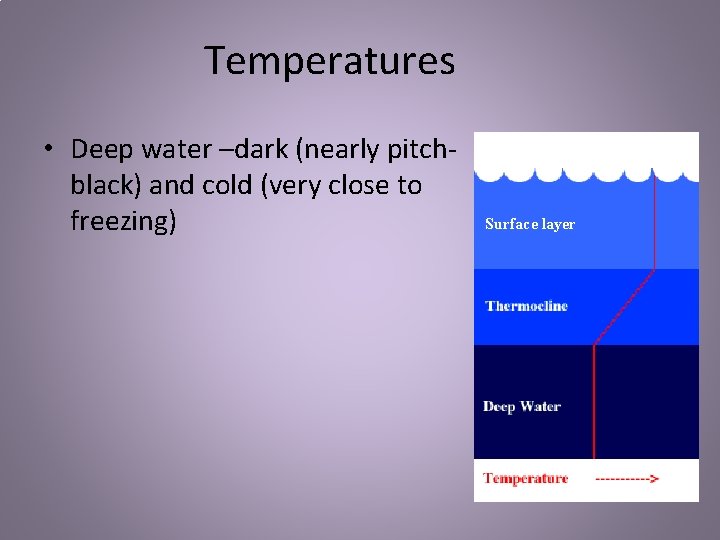
Temperatures • Deep water –dark (nearly pitchblack) and cold (very close to freezing) Surface layer

Temperatures • Relatively few organisms live in the deep • Those that do are some of the most unique organisms on Earth, adapted to live in the cold and dark
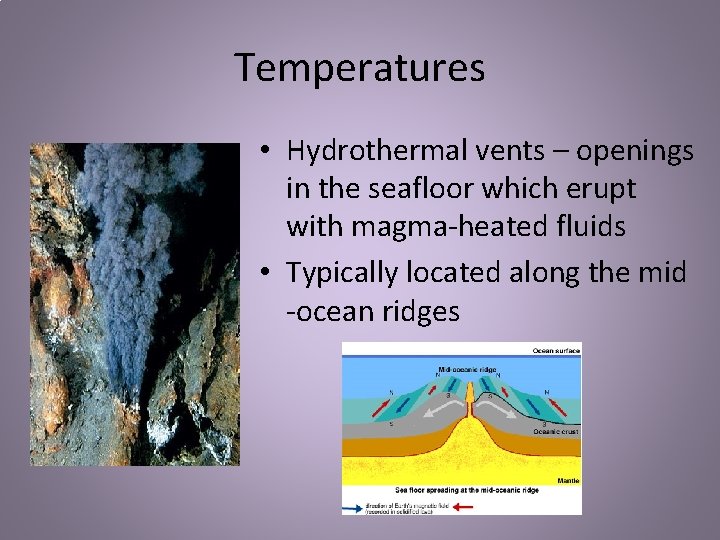
Temperatures • Hydrothermal vents – openings in the seafloor which erupt with magma-heated fluids • Typically located along the mid -ocean ridges

Temperatures • Water can reach 750°F! • Lots of organisms in comparison to the rest of the deep water • These organisms are specially adapted to survive the hot temperatures and chemicals that come out of the vents • Example: Giant Tube Worms
![Salinity and Temperature • Salinity: Salt water is [more / less] dense than freshwater. Salinity and Temperature • Salinity: Salt water is [more / less] dense than freshwater.](http://slidetodoc.com/presentation_image_h2/194bd4089ecc0557e7d66fef52d765c8/image-29.jpg)
Salinity and Temperature • Salinity: Salt water is [more / less] dense than freshwater. Therefore, salt water would [rise above / sink below] fresh water. • Temperature: Cold water is [more / less] dense than warm water. Therefore, cold water would [rise above / sink below] warm water. • Question: Which of the four types of water would float above all the others? A. warm, salty B. warm, fresh C. cold, salty D. cold, fresh



• Get out a sheet of notebook paper • Adaptation – inherited characteristics (characteristics you are born with) that help an organism survive its environment • Example: Where does a polar bear live? • What are some characteristics that help it live there?

• How would you describe deep ocean water? – Warm or cold? – Sunny or dark? • Deep sea creatures have to adapt to live in this environment. We’re going to watch a video about deep sea creatures. As you are watching, write down 5 specific (different from each other) adaptations these creatures have developed that enables them to live in the deep ocean water. • No phones, sleeping, or work from other classes. No cheating! If your tablemate chooses to be off -task during the video while you pay attention, don’t let them copy your paper! Turn in after video.

- Slides: 34