Bell Ringer Name the 4 structures of a
Bell Ringer • Name the 4 structures of a protein. • What types of bonds are between amino acids. Agenda: -Bell Ringer -Setup Lab -Enzyme Notes -Enzyme Lab

Bell Ringer 1. Compare and contrast the two types of inhibitors. Agenda: -Bell Ringer -Enzyme graph analysis -Review Protein Packet -Enzyme Exit Slip -Complete Lab -Study Guide 2. Draw a diagram of an enzymesubstrate interaction. substrate enzyme active site products
Salivary Amylase Graphing Analysis • Due Tuesday! • Must be printed off AND uploaded to i. Network • A screenshot of this must be stapled to your paper!

ENZYMES
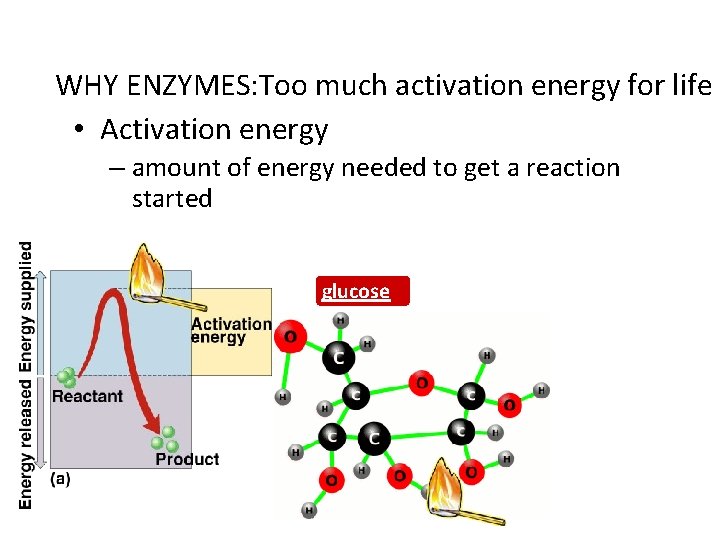
WHY ENZYMES: Too much activation energy for life • Activation energy – amount of energy needed to get a reaction started glucose
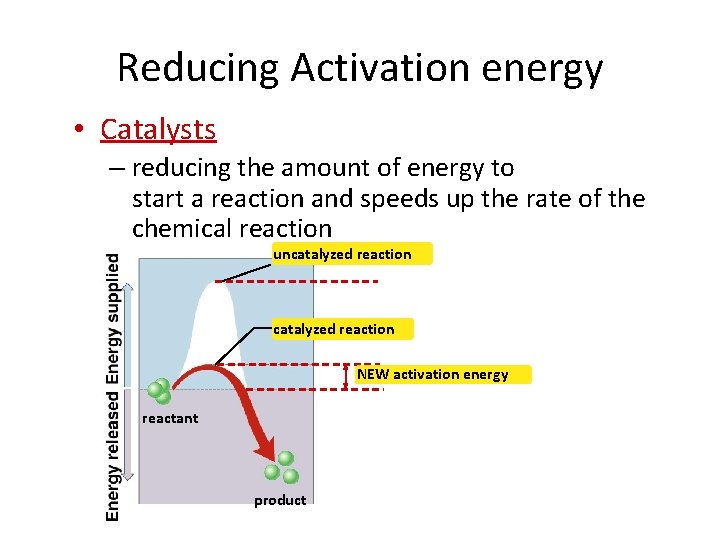
Reducing Activation energy • Catalysts – reducing the amount of energy to start a reaction and speeds up the rate of the chemical reaction uncatalyzed reaction NEW activation energy reactant product
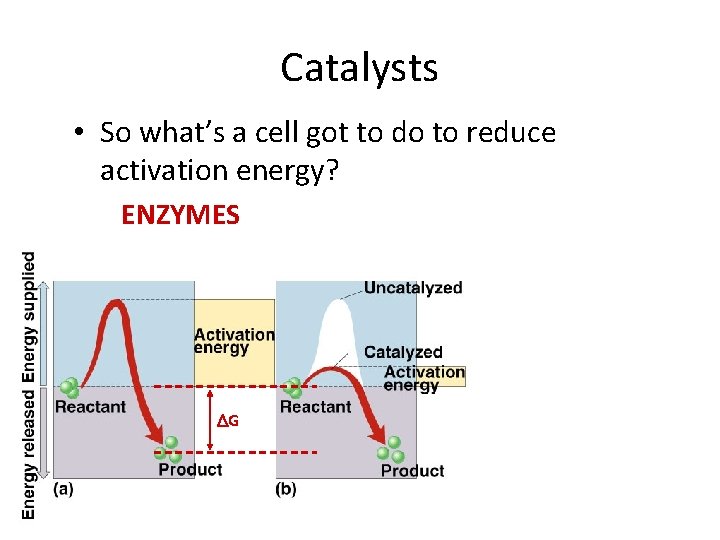
Catalysts • So what’s a cell got to do to reduce activation energy? ENZYMES G
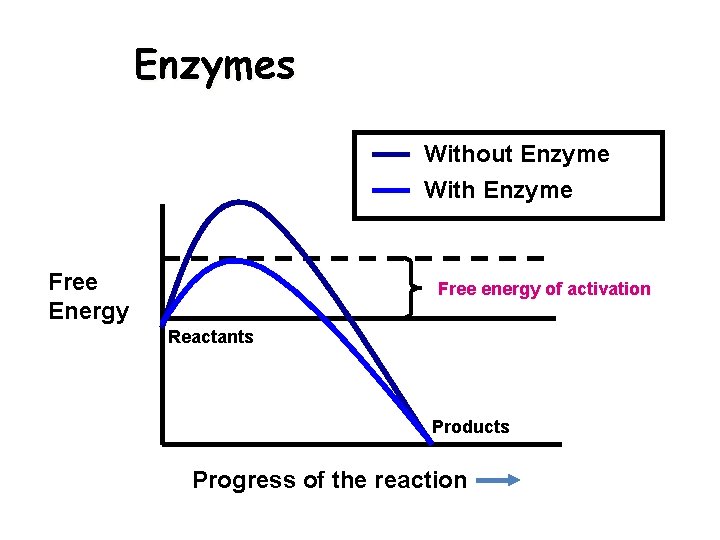
Enzymes Without Enzyme With Enzyme Free Energy Free energy of activation Reactants Products Progress of the reaction
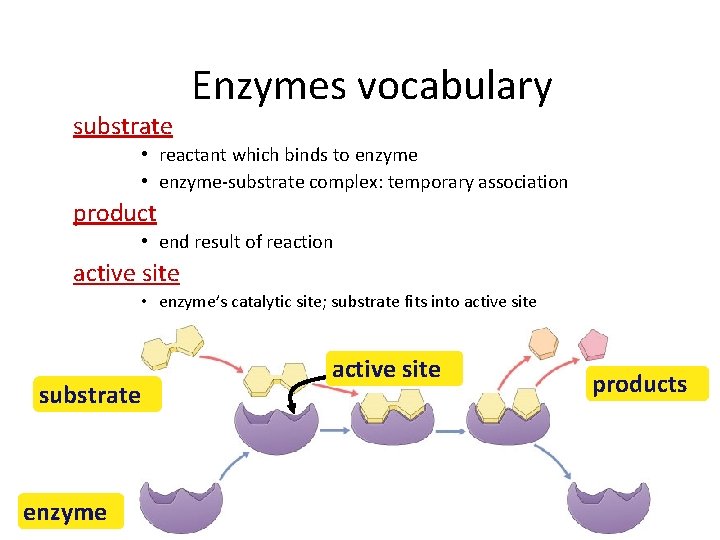
substrate Enzymes vocabulary • reactant which binds to enzyme • enzyme-substrate complex: temporary association product • end result of reaction active site • enzyme’s catalytic site; substrate fits into active site substrate enzyme active site products
Properties of enzymes • Reaction specific – each enzyme works with a specific substrate • chemical fit between active site & substrate • Not consumed in reaction – single enzyme molecule can catalyze thousands or more reactions per second • enzymes unaffected by the reaction • Affected by cellular conditions – any condition that affects protein structure • temperature, p. H, salinity
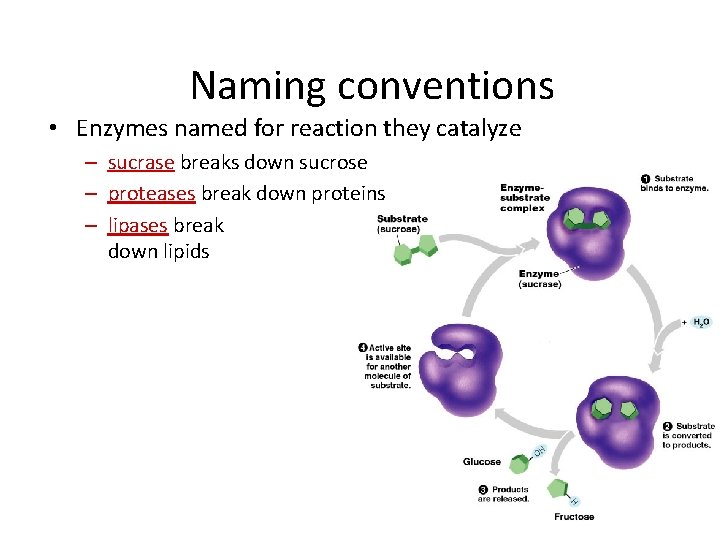
Naming conventions • Enzymes named for reaction they catalyze – sucrase breaks down sucrose – proteases break down proteins – lipases break down lipids
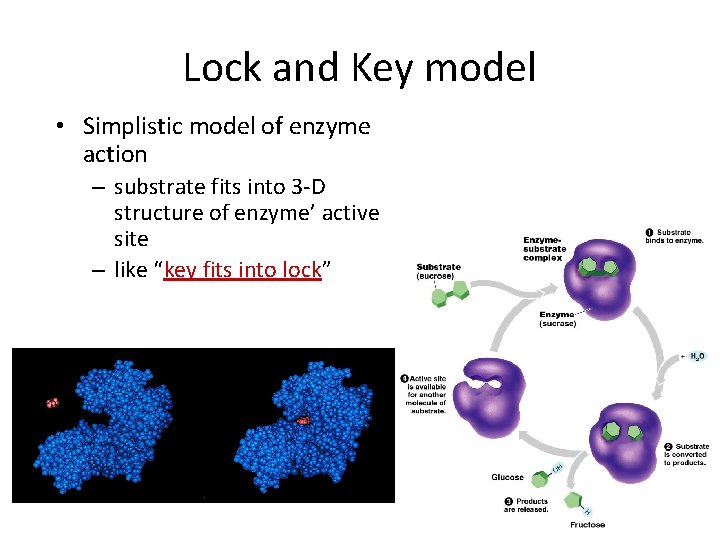
Lock and Key model • Simplistic model of enzyme action – substrate fits into 3 -D structure of enzyme’ active site – like “key fits into lock”
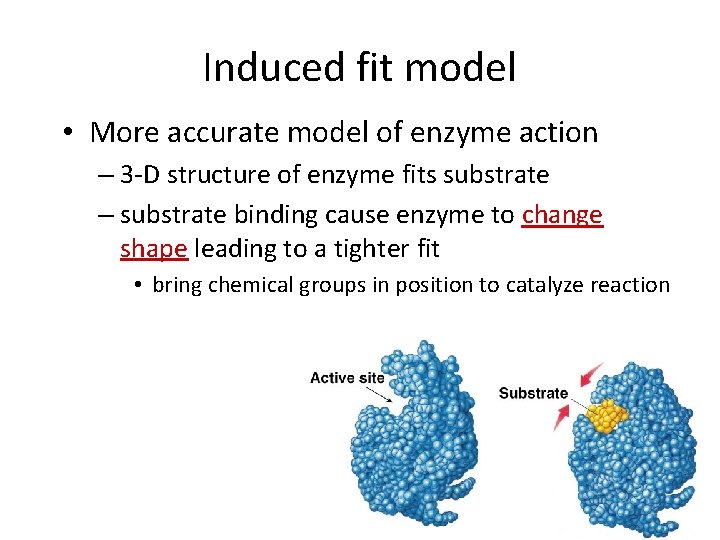
Induced fit model • More accurate model of enzyme action – 3 -D structure of enzyme fits substrate – substrate binding cause enzyme to change shape leading to a tighter fit • bring chemical groups in position to catalyze reaction
Factors that Affect Enzymes 2007 -2008

What Affects Enzyme Activity? • Three factors: 1. Environmental Conditions 2. Cofactors and Coenzymes 3. Enzyme Inhibitors 15
1. Environmental Conditions 1. Extreme Temperature are the most dangerous - high temps may denature (unfold) the enzyme. 2. p. H (most like 6 - 8 p. H near neutral) 3. Ionic concentration (salt ions) 16
2. Cofactors and Coenzymes • Inorganic substances (zinc, iron) and vitamins (respectively) are sometimes need for proper enzymatic activity • Example: Iron must be present in the quaternary structure - hemoglobin in order for it to pick up oxygen. 17
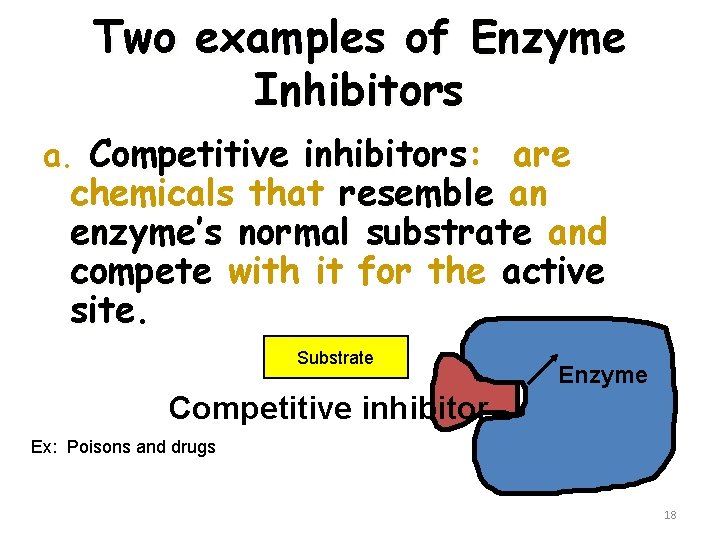
Two examples of Enzyme Inhibitors a. Competitive inhibitors: are chemicals that resemble an enzyme’s normal substrate and compete with it for the active site Substrate Enzyme Competitive inhibitor Ex: Poisons and drugs 18
Example • Ethanol is metabolized (broken down) in body by aldehyde oxidase enzyme • If methanol poisoning happens, ethanol is given. – Ethanol acts as an inhibitor, prohibiting the breakdown of methanol • This would create formaldehyde and formic acid which attack the optic nerve and cause blindness
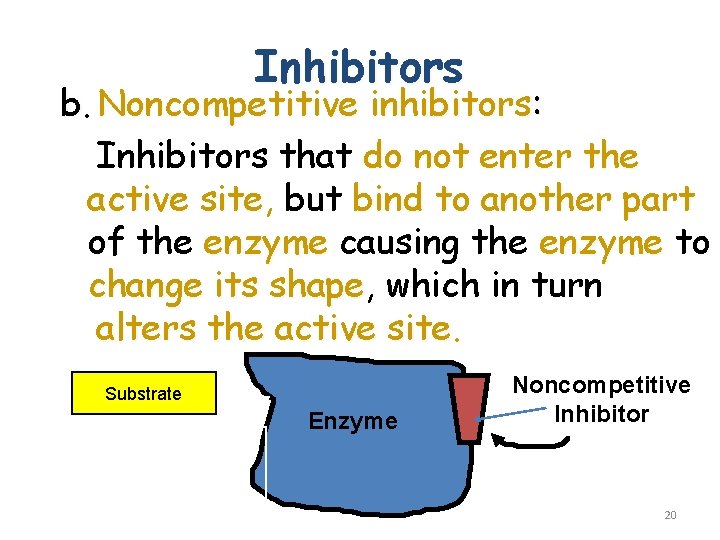
Inhibitors b. Noncompetitive inhibitors: Inhibitors that do not enter the active site, site but bind to another part of the enzyme causing the enzyme to change its shape, shape which in turn alters the active site Substrate active site altered Enzyme Noncompetitive Inhibitor 20
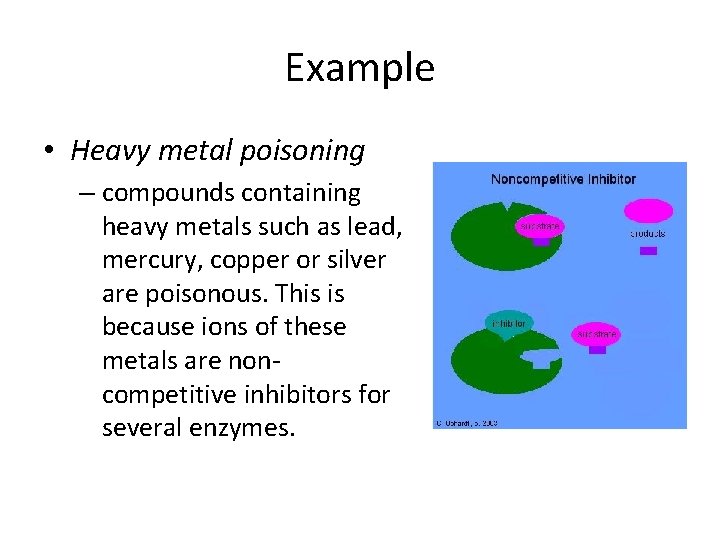
Example • Heavy metal poisoning – compounds containing heavy metals such as lead, mercury, copper or silver are poisonous. This is because ions of these metals are noncompetitive inhibitors for several enzymes.
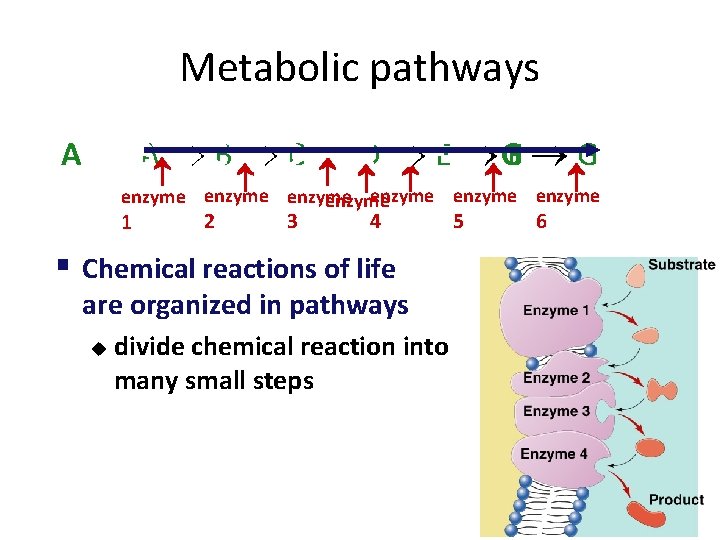
Metabolic pathways A BA CB DC ED FE GF G enzyme enzyme 1 2 3 4 § Chemical reactions of life are organized in pathways u divide chemical reaction into many small steps 5 6

Factors Affecting Enzyme Function • • Enzyme concentration Substrate concentration Temperature p. H Salinity Activators Inhibitors catalase
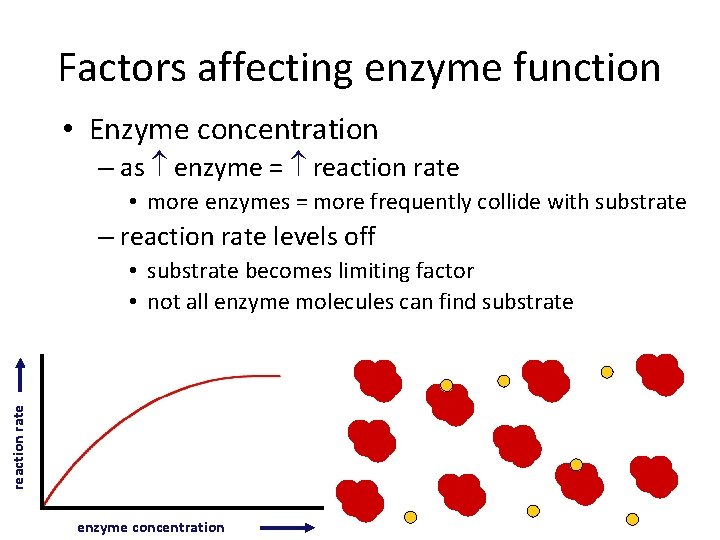
Factors affecting enzyme function • Enzyme concentration – as enzyme = reaction rate • more enzymes = more frequently collide with substrate – reaction rate levels off reaction rate • substrate becomes limiting factor • not all enzyme molecules can find substrate enzyme concentration
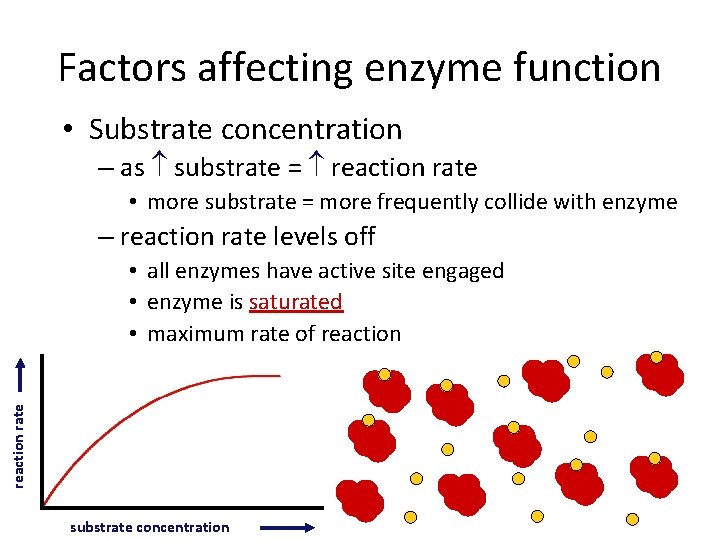
Factors affecting enzyme function • Substrate concentration – as substrate = reaction rate • more substrate = more frequently collide with enzyme – reaction rate levels off reaction rate • all enzymes have active site engaged • enzyme is saturated • maximum rate of reaction substrate concentration
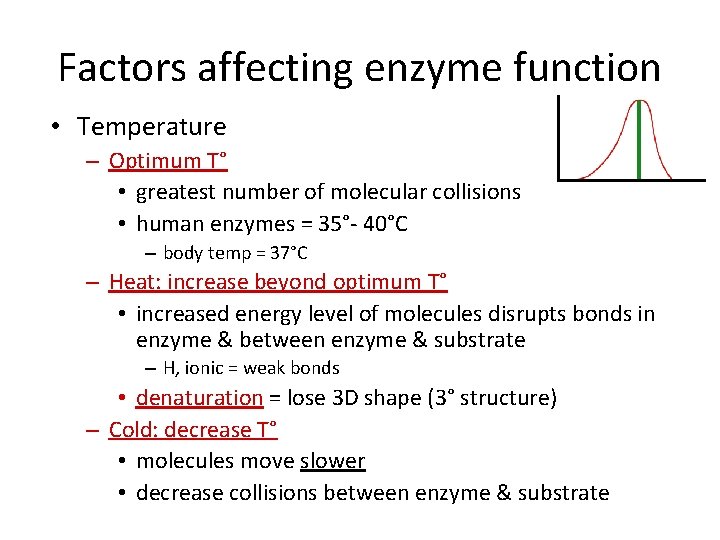
Factors affecting enzyme function • Temperature – Optimum T° • greatest number of molecular collisions • human enzymes = 35°- 40°C – body temp = 37°C – Heat: increase beyond optimum T° • increased energy level of molecules disrupts bonds in enzyme & between enzyme & substrate – H, ionic = weak bonds • denaturation = lose 3 D shape (3° structure) – Cold: decrease T° • molecules move slower • decrease collisions between enzyme & substrate
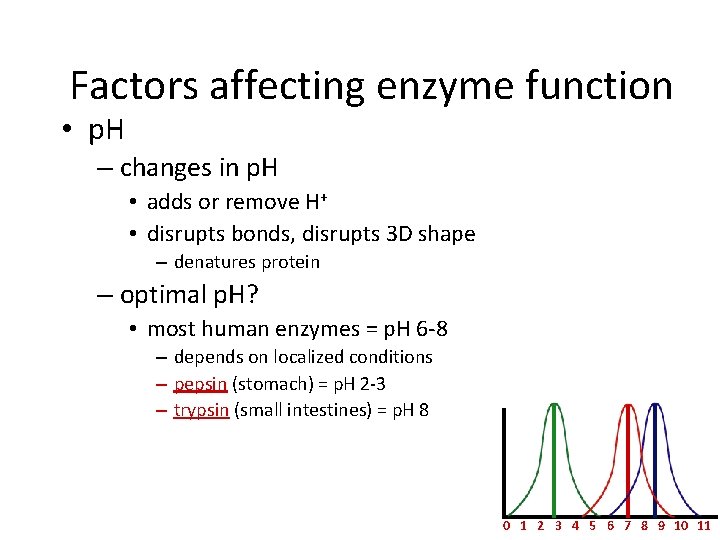
Factors affecting enzyme function • p. H – changes in p. H • adds or remove H+ • disrupts bonds, disrupts 3 D shape – denatures protein – optimal p. H? • most human enzymes = p. H 6 -8 – depends on localized conditions – pepsin (stomach) = p. H 2 -3 – trypsin (small intestines) = p. H 8 0 1 2 3 4 5 6 7 8 9 10 11
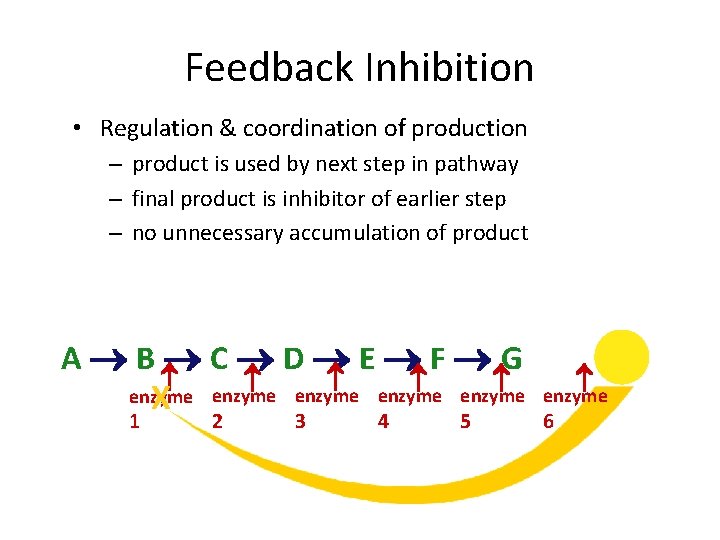
Feedback Inhibition • Regulation & coordination of production – product is used by next step in pathway – final product is inhibitor of earlier step – no unnecessary accumulation of product X A B C D E F G enzyme enzyme 1 2 3 4 5 6

Enzyme Lab • 1 m. L = 20 drops
- Slides: 30