Bell Ringer April 18 th What type of
Bell Ringer April 18 th • What type of solutions are dissolved in water? • What type of compounds usually produce ions when dissolved?

ACIDS AND BASES

• https: //www. youtube. com/watch? v=IAJs. ZWhj 6 GI

Acids and Bases When a substance dissolves in water it makes an aqueous solution. Solutions can be sorted by whether they are: acid, basic (alkali) or neutral.

ACIDS – Taste sour – Turn litmus paper red – Neutralize bases – Acids are made of one or more H+ atoms and a negative ion – Ex. HCl – Produces H+ ions when dissolved in water – Are corrosive

Acids There are many acids present in our everyday lives. Lemon juice contains citric acid, and vinegar contains ethanoic acid. Some strong acids are hydrochloric acid, sulphuric acid and nitric acid. Some weak acids are ethanoic acid, citric acid and carbonic acid.

BASES • • Taste bitter Turn litmus paper blue Neutralize acids Bases are made of metals combined with hydroxide ions (OH-) Ex. Na. OH Produce OH- when dissolved in water Feel slippery or “soapy” Are corrosive

Bases Alkalis are present in many cleaning substances used in our homes. Kitchen cleaners are alkaline because they contain ammonia or sodium hydroxide, which attack grease. Calcium hydroxide and sodium hydroxide are strong alkalis. The most recognizable and common weak alkali is ammonia.
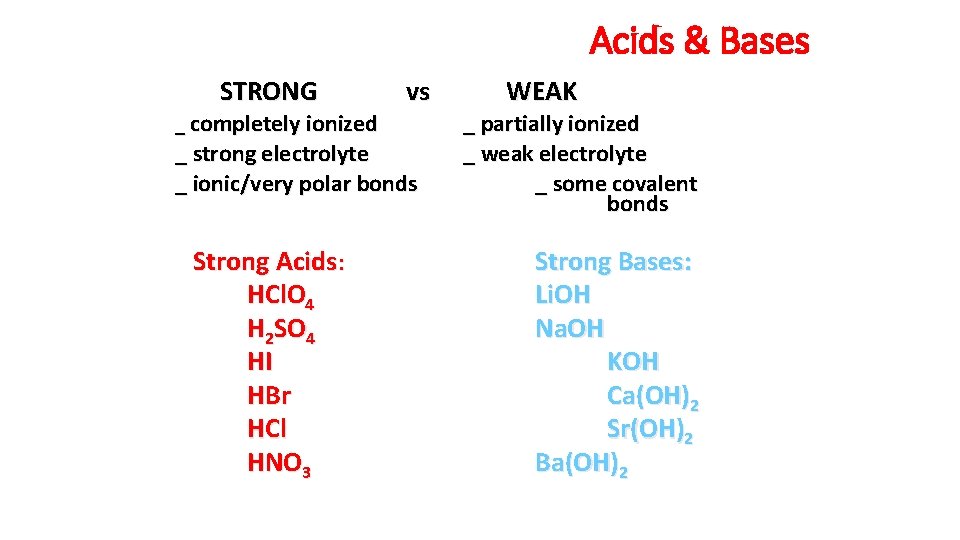
Acids & Bases STRONG _ completely ionized vs _ strong electrolyte _ ionic/very polar bonds Strong Acids: HCl. O 4 H 2 SO 4 HI HBr HCl HNO 3 WEAK _ partially ionized _ weak electrolyte _ some covalent bonds Strong Bases: Li. OH Na. OH KOH Ca(OH)2 Sr(OH)2 Ba(OH)2
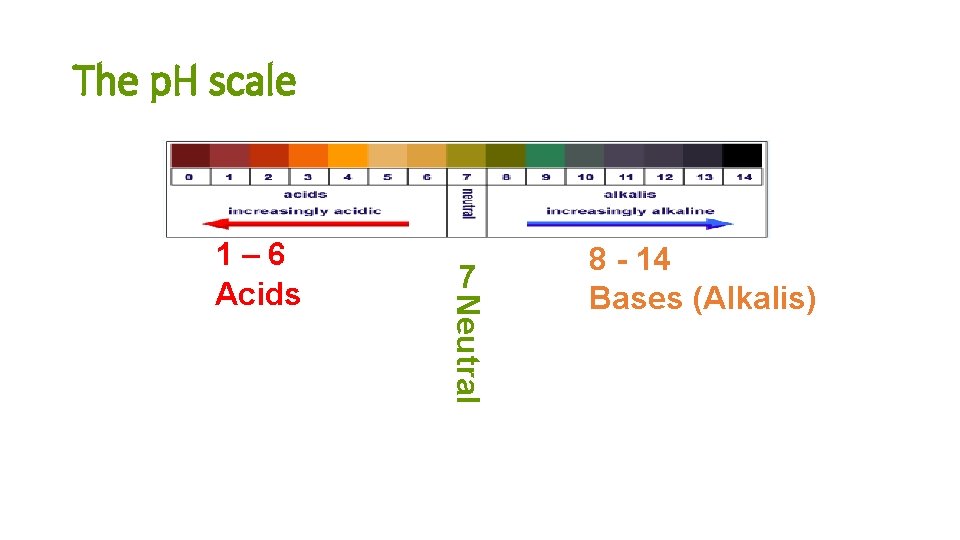
The p. H scale 7 Neutral 1– 6 Acids 8 - 14 Bases (Alkalis)

• https: //www. youtube. com/watch? v=Jfa 2 wxp. JJBA

Exit Ticket April 18 th • Are they acids or bases? 1) 2) 3) 4) 5) p. H=9 Ca(OH)2 HBr p. H= 3 p. H=7 - Why is p. H important to monitor in the body? How does acids and bases effect this?
- Slides: 12