Behavior Properties of Solids Liquids Gases Pages 400

Behavior & Properties of Solids, Liquids & Gases Pages 400 -433

What are 3 things you know about the behavior and properties of solids, liquids & gases?

Learning Goals- Sections 12. 1 and 12. 2 Apply the kinetic-molecular theory to explain the behavior or gases. Describe how mass affects the rates of diffusion and effusion. Explain how gas pressure is measured and calculate the partial pressure of a gas. Describe what intramolecular forces are and how they affect the properties of a substance.
GASES & Kinetic molecular Theory Section 12. 1; pages 402 -410
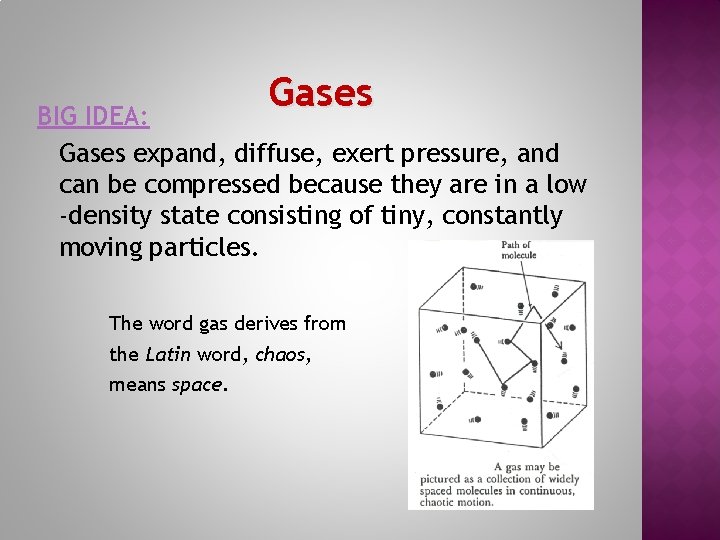
Gases BIG IDEA: Gases expand, diffuse, exert pressure, and can be compressed because they are in a low -density state consisting of tiny, constantly moving particles. The word gas derives from the Latin word, chaos, means space.

Gases are compressible. Great amounts of space exist between gas particles. Compression reduces the empty spaces between particles.

Kinetic-molecular theory explains the different properties of solids, liquids, and gases. The kinetic-molecular theory describes the behavior of matter in terms of: Particle size 2. Motion 3. Energy 1. The word kinetic derives from the Greek word meaning to move.

Is based upon the following properties of gases: 1. Particle Size Gases consist of small particles separated by empty space. The volume of gas particles is assumed to be zero. Gas particles are assumed to experience no significant attractive or repulsive forces.

Is based upon the following properties of gases: 2. Particle Motion Gas particles are in constant random motion. Gas particles move in a straight line until they collide with other particles or the walls of the container. The collisions are assumed to be elastic. There is no (kinetic) energy lost in an elastic collision. Kinetic energy may be transferred between two colliding particles.
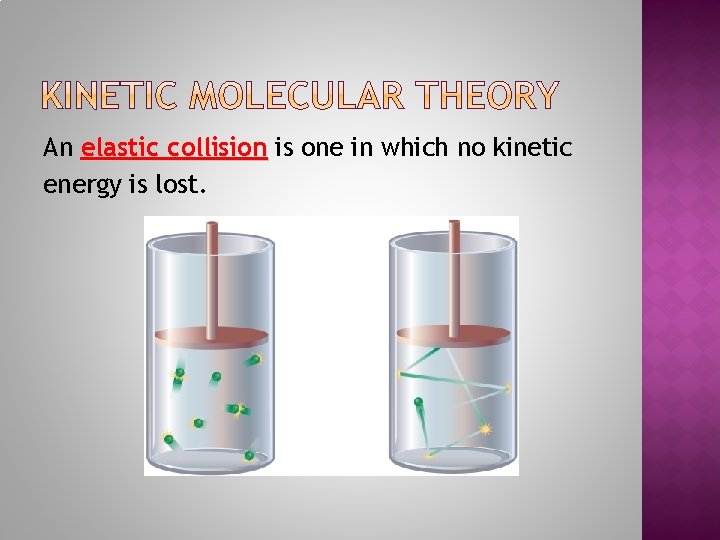
An elastic collision is one in which no kinetic energy is lost.

Is based upon the following properties of gases: 3. Particle Energy Kinetic Energy of a particle depends on Mass and Velocity. All particles of the same gas will have the same mass but not the same velocity and so will not have the same kinetic energy.
Is based upon the following properties of gases: 3. Particle Energy Temperature is a measure of the average kinetic energy of the particles in a sample of matter.
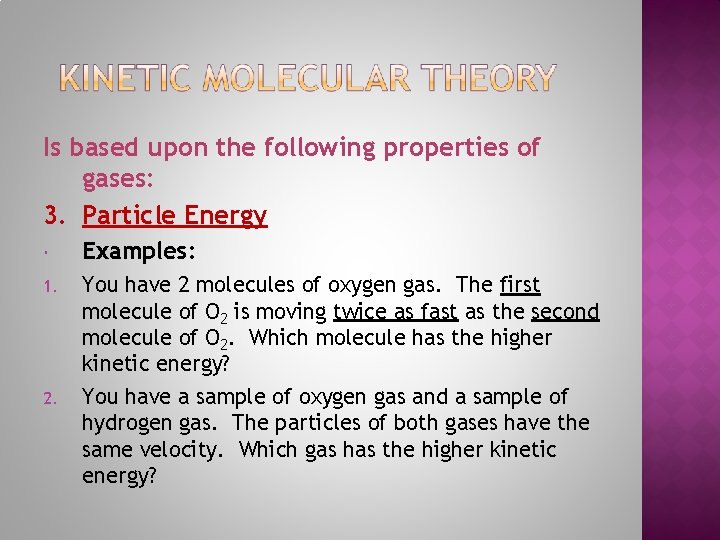
Is based upon the following properties of gases: 3. Particle Energy Examples: 1. You have 2 molecules of oxygen gas. The first molecule of O 2 is moving twice as fast as the second molecule of O 2. Which molecule has the higher kinetic energy? You have a sample of oxygen gas and a sample of hydrogen gas. The particles of both gases have the same velocity. Which gas has the higher kinetic energy? 2.
1. 2. 3. 4. What 3 properties is the kinetic molecular theory of gases based upon? You have 2 samples of nitrogen gas. Sample #1 has twice as much energy as sample #2. Which sample of nitrogen gas has a faster average velocity? You have 2 samples of hydrogen gas. Sample #2 has a higher temperature than sample #1. Which sample of hydrogen gas has a higher kinetic energy? You have 2 samples of unknown gases moving at the same average velocity. Sample #1 has a lower kinetic energy than sample #2. Which sample of gas has the higher mass?

Explaining the Behavior of Gases have a low density due to the large amount of empty space between particles. The density of gases is less than the density for liquids and solids. Cl 2 gas has a density of 2. 95 X 10 -3 g/m. L Solid gold, Au, has a density of 19. 2 g/m. L (6500 times greater) Why? - There are fewer chlorine molecules in the same amount of volume than for gold. How do you explain this using the kinetic molecular theory?
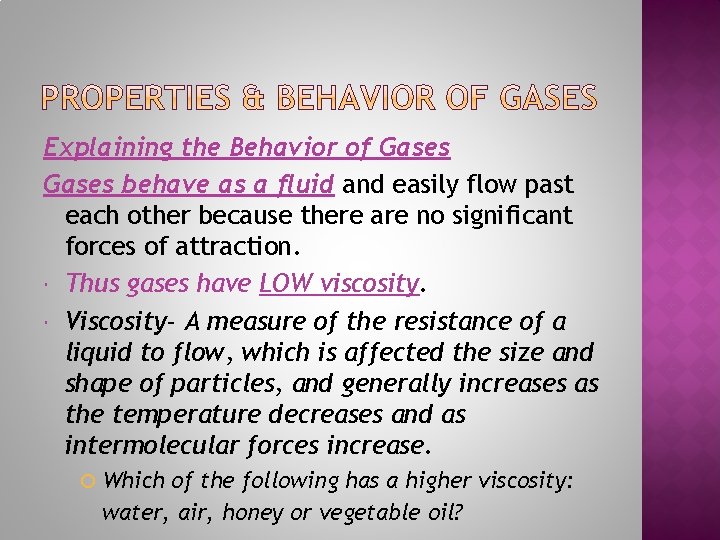
Explaining the Behavior of Gases behave as a fluid and easily flow past each other because there are no significant forces of attraction. Thus gases have LOW viscosity. Viscosity- A measure of the resistance of a liquid to flow, which is affected the size and shape of particles, and generally increases as the temperature decreases and as intermolecular forces increase. Which of the following has a higher viscosity: water, air, honey or vegetable oil?

Explaining the Behavior of Gases particles flow past each other easily and are in constant random motion. This random motion causes gases to mix until they are evenly distributed. Diffusion is the movement of one material through another. Think of an odor spreading throughout a room. Effusion is the escaping of a gas through a tiny opening.

Diffusion Versus Effusion
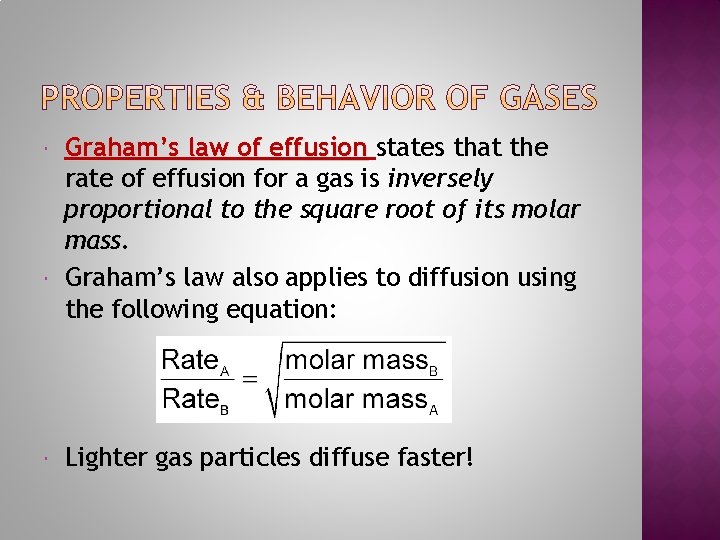
Graham’s law of effusion states that the rate of effusion for a gas is inversely proportional to the square root of its molar mass. Graham’s law also applies to diffusion using the following equation: Lighter gas particles diffuse faster!
Graham’s law of effusion states that the rate of effusion for a gas is inversely proportional to the square root of its molar mass. Examples: Which gas will diffuse faster, NH 3 or Cl 2? Which gas will diffuse faster, CO 2 or CH 4?

Pressure is defined as force per unit area. Gas particles exert pressure when they collide with the walls of their container. Atmospheric (or air) pressure is the pressure exerted by the earth’s atmosphere which extends hundreds of kilometers into space. The earth’s atmospheric pressure will vary by altitude. Why? How do we measure air pressure?
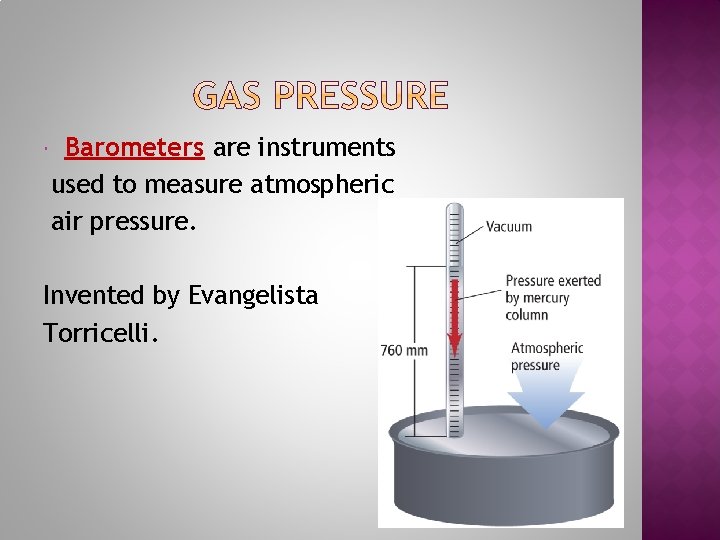
Barometers are instruments used to measure atmospheric air pressure. Invented by Evangelista Torricelli.

Review of Kinetic Molecular Theory (KMT): 1. What 3 observations of matter are used to describe its behavior? 2. What happens to the kinetic energy of a gas when you : Increase temperature of the gas? 2. Decrease mass of the gas? 3. Increase velocity of gas particles? 1. 3. How can you use KMT to explain the following: 1. 2. 3. Why a gas has such a low density? Why solids are more dense than gases? Why gases are compressible?

Diffusion vs. Effusion: 4. What is the difference? 5. What determines how fast a gas will diffuse or effuse? 6. Which pairs of gases will diffuse/effuse the fastest? 1. 2. 7. Propane, C 3 H 8, or CO 2 HCl or Cl 2 You have a collision between a tank truck carrying radioactive I 2 gas and another truck carrying deadly mustard gas, C 4 H 8 Cl 2 S. Which gas do you act to contain first?

8. As a gas is expanded in a cylinder: 1. What happens to its mass? 2. What happens to the distance between the gas molecules? 3. What happens to the number of gas molecules? 4. What happens to the density of the gas?
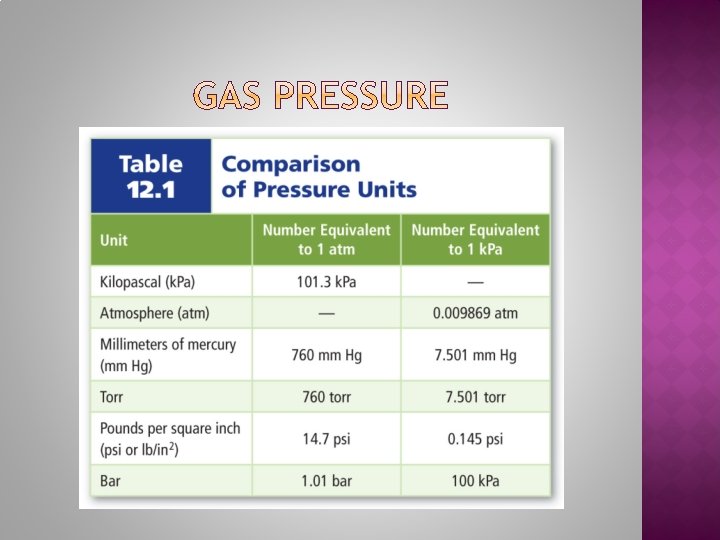

1. 2. 3. 4. 5. How do gases exert pressure? What is a barometer? What is the atmospheric pressure at sea level? What is the pressure of a gas in kilopascal (k. Pa) if it is at 2 atm of pressure? Convert the pressure of a gas at 5 atm to torr
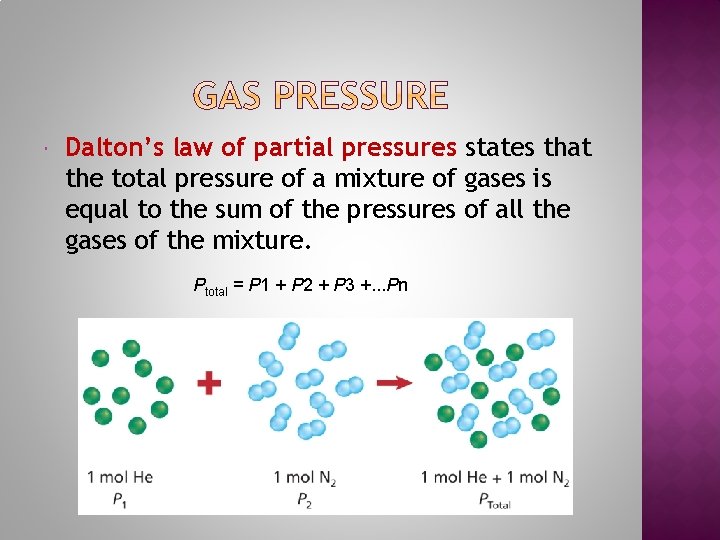
Dalton’s law of partial pressures states that the total pressure of a mixture of gases is equal to the sum of the pressures of all the gases of the mixture. Ptotal = P 1 + P 2 + P 3 +. . . Pn

1. Find the total pressure for a mixture that contains 4 gases with partial pressures of 5. 00 k. Pa, 4. 56 k. Pa, 3. 02 k. Pa, and 1. 20 k. Pa. 2. Find the partial pressure of CO 2 in a gas mixture with a total pressure of 30. 4 k. Pa if the partial pressures of the other two gases in the mixture are 16. 5 k. Pa and 3. 7 k. Pa. 3. A mixture of oxygen, carbon dioxide, and nitrogen has a total pressure of 0. 97 atm. What is the partial pressure of O 2 if the partial pressure of CO 2 is 0. 70 atm and the partial pressure of N 2 is 0. 12 atm?

Forces of Attraction Pages 411 -414

Why are some substances gases at room temperature while others are liquids and solids? Answer: there are various forces of attraction between and among molecules. BIG IDEA: There are 3 types of intermolecular forces: 1. dispersion forces 2. dipole-dipole forces 3. hydrogen bonds. These 3 forces determine a substance’s state of matter at a given temperature.
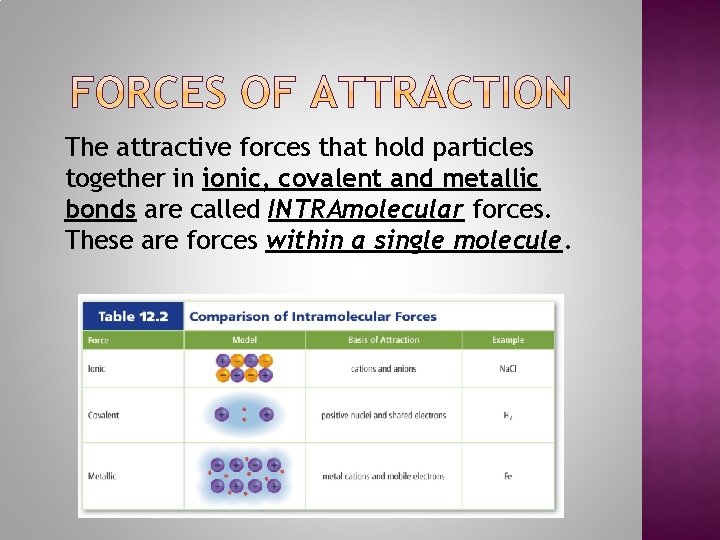
The attractive forces that hold particles together in ionic, covalent and metallic bonds are called INTRAmolecular forces. These are forces within a single molecule.
There are other forces called INTERmolecular forces. These are forces between many molecules. There are 3 types of INTERmolecular forces: 1. 2. 3. Dispersion forces Dipole-dipole forces Hydrogen bonds INTERmolecular forces are WEAKER than INTRAmolecular forces
Intermolecular forces occur because molecules often have one end which is slightly positive and one end which is slightly negative. This is due to the electronegativity differences between the bonded atoms. Review- electronegativity The relative ability of an atom to attract the electrons in a chemical bond. Remember F has the highest electronegativity and Cs has the lowest electronegativity.
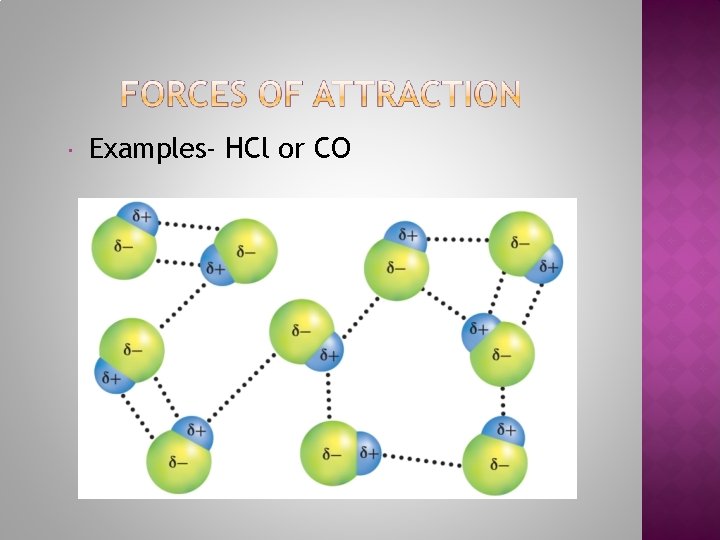
Examples- HCl or CO

Hydrogen bonding A special type of dipole-dipole attractive force between molecules containing a HYDROGEN ATOM and either a fluorine, oxygen, nitrogen or chlorine. Examples: HCl, NH 3, HF and H 2 O
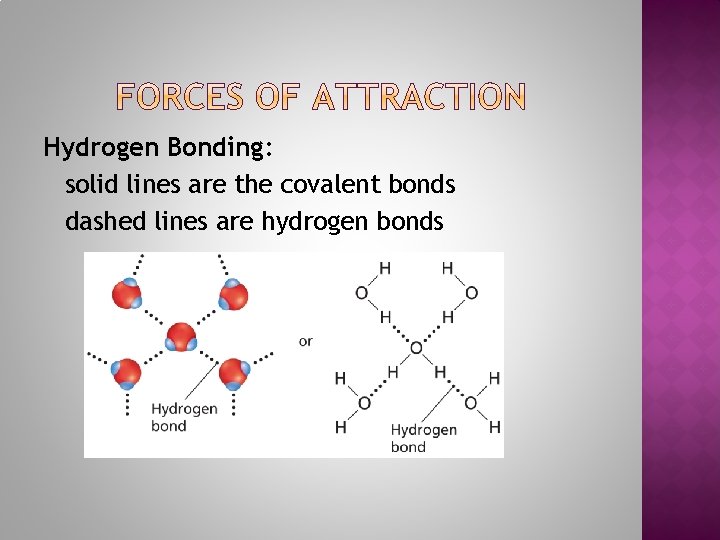
Hydrogen Bonding: solid lines are the covalent bonds dashed lines are hydrogen bonds
Liquids and Solids

BIG IDEA: The particles in solids and liquids have a limited range of motion and are not easily compressed. Forces of attraction keep molecules closely packed in a fixed volume, but not in a fixed position. Liquids are much denser than gases because of the stronger intermolecular forces holding the particles together.

Fluidity is the ability to flow and diffuse; liquids and gases are fluids. Viscosity is a measure of the resistance of a liquid to flow and is determined by the type of intermolecular forces, size and shape of particles, and temperature.
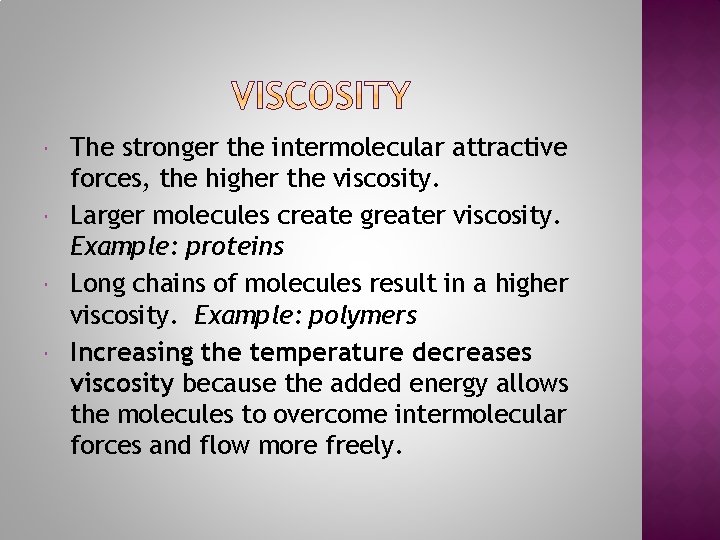
The stronger the intermolecular attractive forces, the higher the viscosity. Larger molecules create greater viscosity. Example: proteins Long chains of molecules result in a higher viscosity. Example: polymers Increasing the temperature decreases viscosity because the added energy allows the molecules to overcome intermolecular forces and flow more freely.

Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surfactants are compounds that lower the surface tension of water.
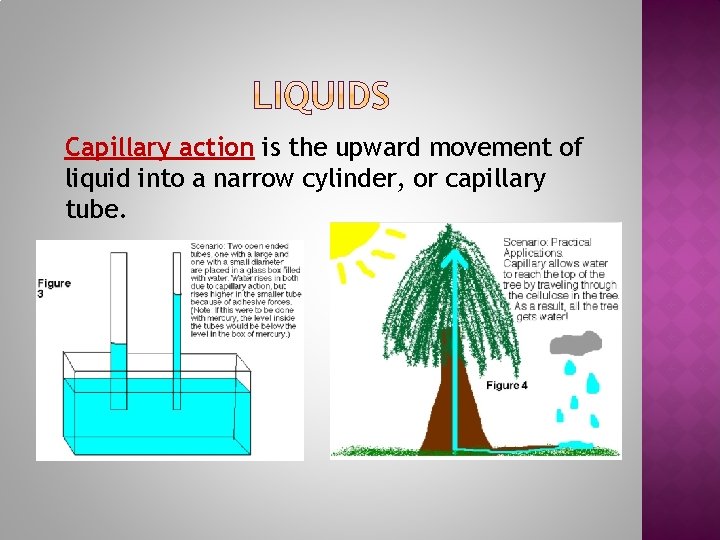
Capillary action is the upward movement of liquid into a narrow cylinder, or capillary tube.

Solids contain particles with strong attractive intermolecular forces. Particles in a solid vibrate in a fixed position. Most solids are more dense than liquids. EXCEPTION-(solid) ice is less dense than (liquid) water.
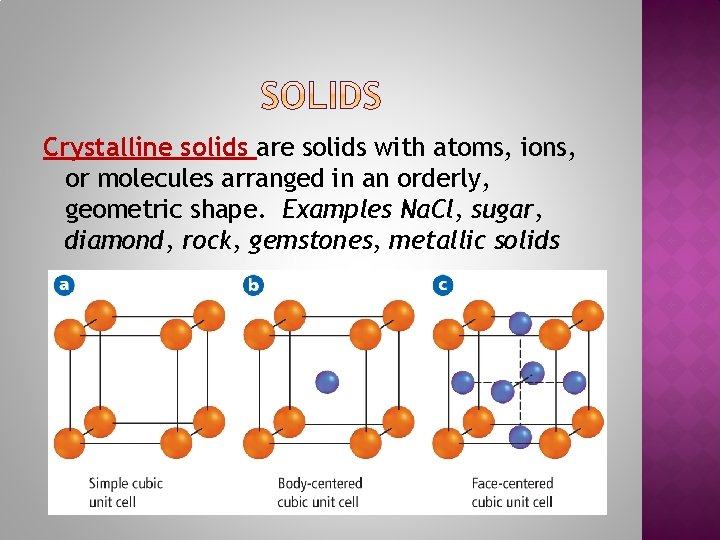
Crystalline solids are solids with atoms, ions, or molecules arranged in an orderly, geometric shape. Examples Na. Cl, sugar, diamond, rock, gemstones, metallic solids

Amorphous solids are solids in which the particles are not arranged in a regular, repeating pattern. Amorphous solids form when molten material cools quickly. Examples: cooled lava, glass, rubber and many kinds of plastics

Phase Changes

Your mission for today: 1. Can you name the 6 possible phase changes or transitions? 2. Can you explain if a particular phase change requires the removal or addition of heat? 3. Can you interpret a phase diagram?

BIG IDEA: Matter changes phase when energy is added or removed. What happens when heat energy is added to ice? Is this a chemical or a physical change? Melting occurs when heat flows into a solid object.
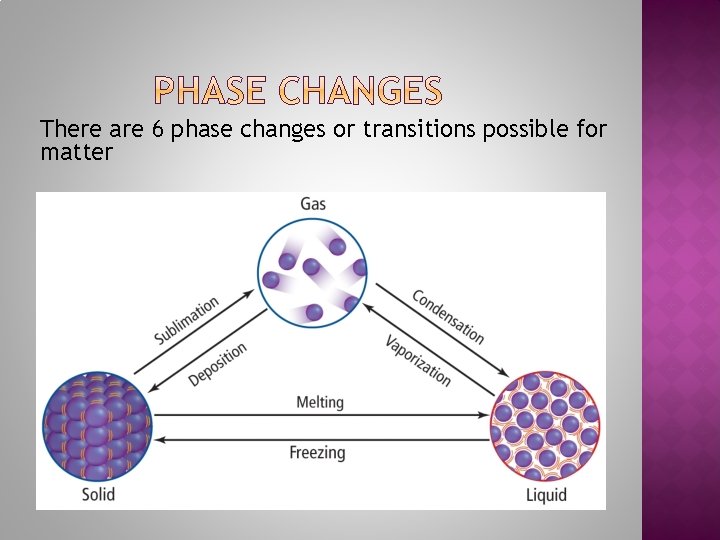
There are 6 phase changes or transitions possible for matter
The addition of heat energy is an ENDOTHERMIC process Melting Vaporization/boiling sublimation The removal of heat energy is an EXOTHERMIC process Freezing Condensation Deposition

The melting point of a crystalline solid is the temperature at which the forces holding the crystal lattice together are broken and it becomes a liquid. Vaporization is the process by which a liquid changes to a gas or vapor. Evaporation is vaporization only at the surface of a liquid. Sublimation is the process by which a solid changes into a gas without becoming a liquid.
The boiling point is the temperature at which the vapor pressure of a liquid equals the atmospheric pressure. The freezing point is the temperature at which a liquid is converted into a crystalline solid. The process by which a gas or vapor becomes a liquid is called condensation. Deposition is the process by which a gas or vapor changes directly to a solid, and is the reverse of sublimation.
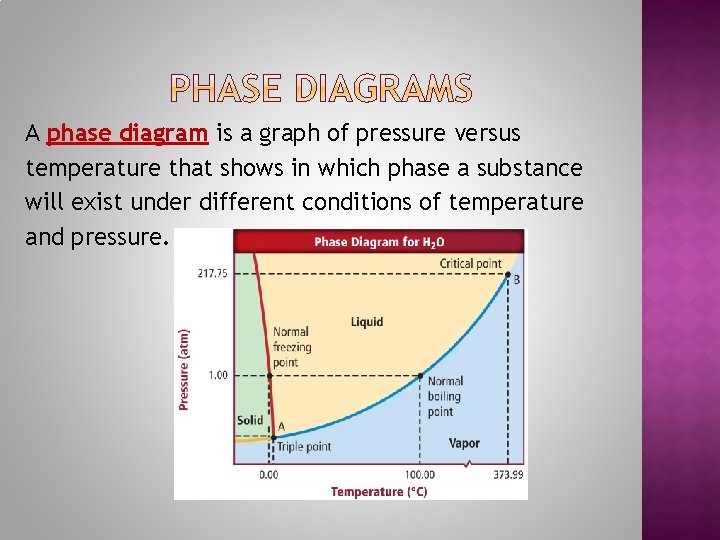
A phase diagram is a graph of pressure versus temperature that shows in which phase a substance will exist under different conditions of temperature and pressure.
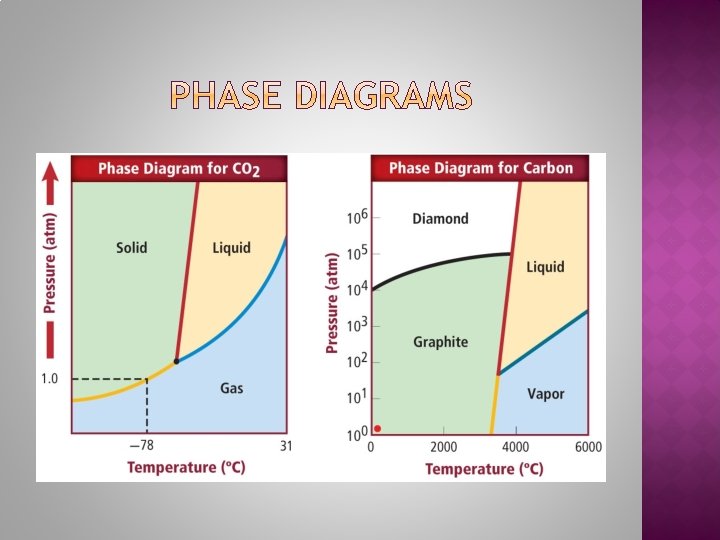

The triple point is the point on a phase diagram that represents the temperature and pressure at which all three phases of a substance can coexist. The critical point is the point above which a gas can no longer be changed back into a liquid.
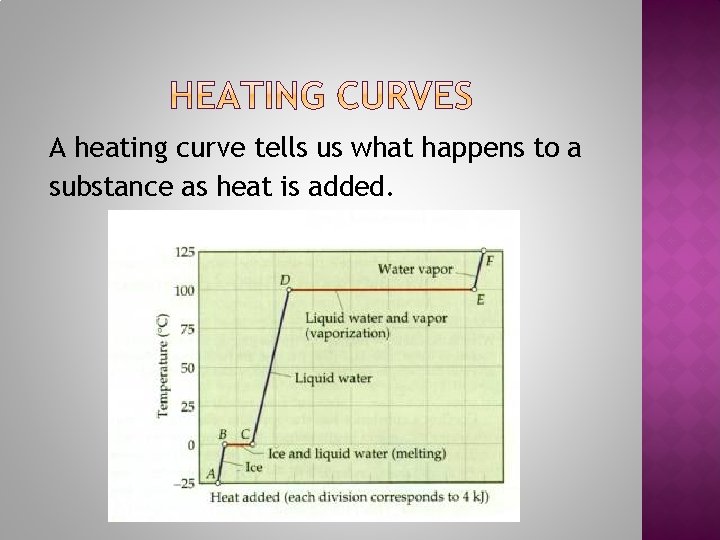
A heating curve tells us what happens to a substance as heat is added.
- Slides: 57