Behavior of Liquids and Gases Thermal Expansion As

Behavior of Liquids and Gases

Thermal Expansion As temperature rises, particles move faster and faster. Thermal Expansion is an increase in the size of a substance when the temperature is increased. Substances also contract when they cool. Examples: Liquid in a thermometer and air expanding in a hot air balloon.

Properties of Fluids Buoyancy is the ability of a fluid—a liquid or a gas– to exert an upward force on an object immersed in it. If the buoyant force is equal to the object’s weight, the object will float.
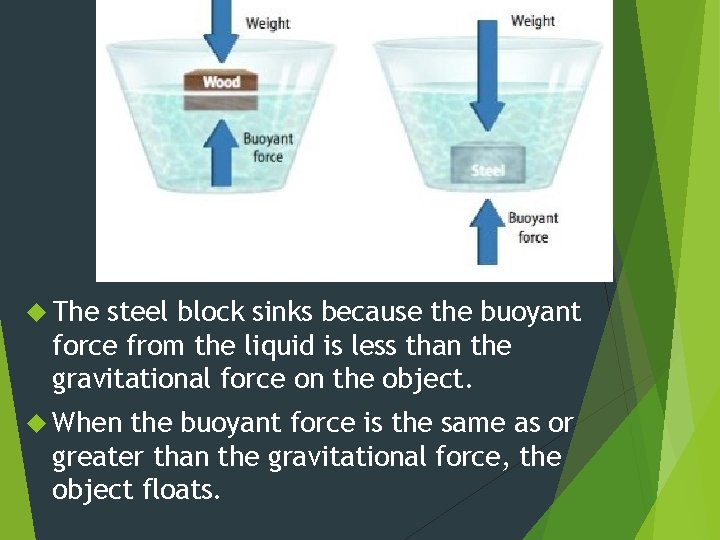
The steel block sinks because the buoyant force from the liquid is less than the gravitational force on the object. When the buoyant force is the same as or greater than the gravitational force, the object floats.
Density and Buoyancy Density is mass per unit of volume One way to know whether an object will float is to compare its density to the density of the fluid in which it is placed. An object floats if its density is less than that of a fluid

Pressure is force exerted per unit area. The SI unit of pressure is the pascal (Pa). Blaise Pascal discovered that pressure applied to a fluid is transmitted throughout the fluid. Pressure in=Pressure out Pascal’s Principle: Input Force (N) = Output Force (N) Input Area (m 2) = Output Area (m 2) Fin = Fout Ain = Aout
Bernoulli’s Principle Daniel Bernoulli discovered that fluid velocity increases when the flow of fluid is restricted. According to Bernoulli’s Principle, as the velocity of a fluid increases, the pressure exerted by that fluid decreases.

Viscosity Another property exhibited by a fluid is its tendency to flow. While all fluids flow, they vary in the rates at which they flow. Viscosity is the resistance of a fluid to flowing. High viscosity = High resistance to flow Examples: Syrup


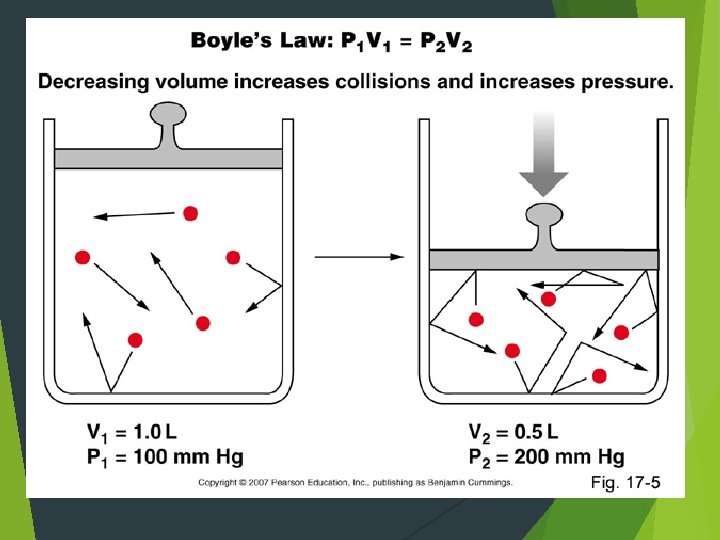
Behavior of Gases A gas completely fills its container. Collisions amongst gas particles and the container wall cause the gas to exert pressure on the container. The pressure from a gas depends on how often its particles strike the walls of the container. If you squeeze gas into a smaller space, its particles will strike the walls more often, causing increased pressure. The opposite is also true.
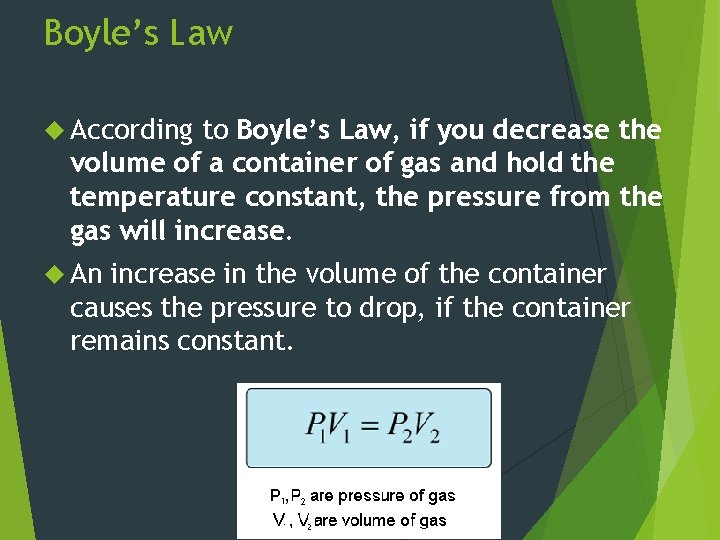
Boyle’s Law According to Boyle’s Law, if you decrease the volume of a container of gas and hold the temperature constant, the pressure from the gas will increase. An increase in the volume of the container causes the pressure to drop, if the container remains constant.
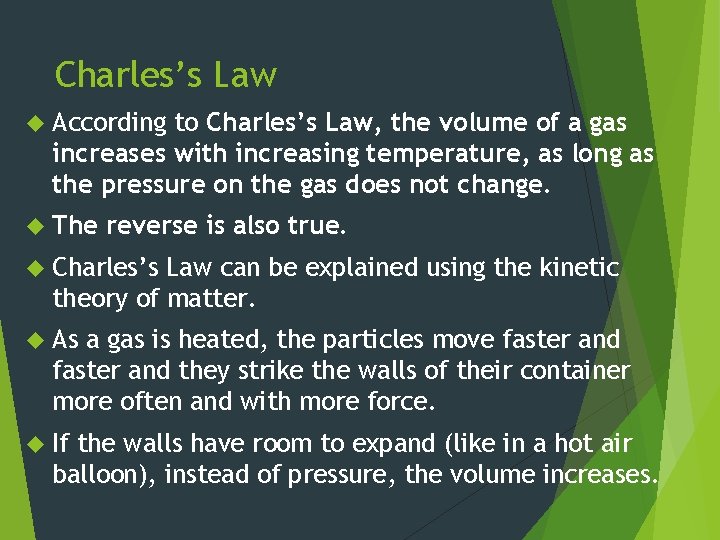
Charles’s Law According to Charles’s Law, the volume of a gas increases with increasing temperature, as long as the pressure on the gas does not change. The reverse is also true. Charles’s Law can be explained using the kinetic theory of matter. As a gas is heated, the particles move faster and they strike the walls of their container more often and with more force. If the walls have room to expand (like in a hot air balloon), instead of pressure, the volume increases.
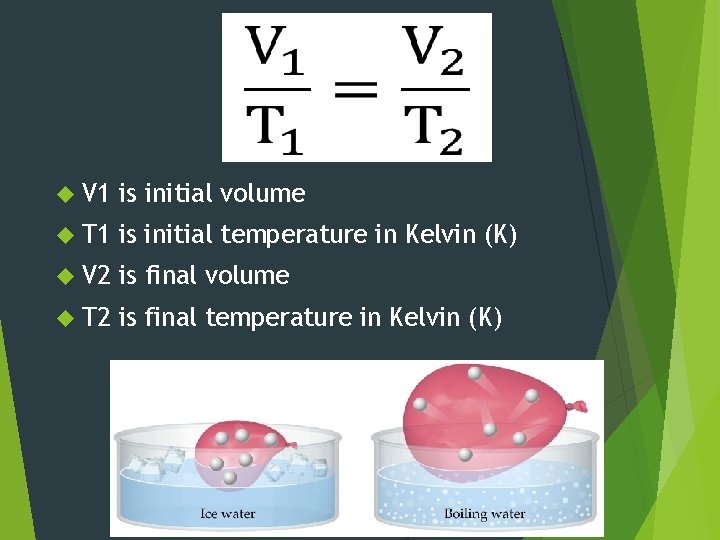
V 1 is initial volume T 1 is initial temperature in Kelvin (K) V 2 is final volume T 2 is final temperature in Kelvin (K)
- Slides: 14