Because learning changes everything Chapter 2 The Chemistry

Because learning changes everything. ® Chapter 2 The Chemistry of Life ANATOMY & PHYSIOLOGY The Unity of Form and Function NINTH EDITION KENNETH S. SALADIN © 2021 Mc. Graw Hill. All rights reserved. Authorized only for instructor use in the classroom. No reproduction or further distribution permitted without the prior written consent of Mc. Graw Hill.
Introduction Biochemistry • The study of the molecules that compose living organisms • Carbohydrates, fats, proteins, and nucleic acids • Useful for understanding cellular structures, basic physiology, nutrition, and health © Mc. Graw Hill 2
2. 1 Atoms, Ions, and Molecules Expected Learning Outcomes: • Identify the elements of the body from their symbols. • Distinguish between elements and compounds. • State the functions of minerals in the body. • Explain the basis for radioactivity and the types and hazards of ionizing radiation. • Distinguish between ions, electrolytes, and free radials. • Define the types of chemical bonds. © Mc. Graw Hill 3
The Chemical Elements 1 Element • Simplest form of matter to have unique chemical properties Atomic number (for an element) • Number of protons in the nucleus • Periodic table • Elements arranged by atomic number • Elements represented by one- or two-letter symbols 24 elements have biological roles • 6 elements = 98. 5% of body weight • Oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus • Trace elements • Present in minute amounts, but play vital roles © Mc. Graw Hill 4
The Chemical Elements 2 Minerals • Inorganic elements extracted from soil by plants and passed up food chain to humans • Constitute about 4% of body weight • Ca and P make up about 3% • Remaining 1% is mainly Cl, Mg, K, Na, and S • Important for body structure (Ca crystals in teeth, bones, etc. ) • Important for enzyme function • Electrolytes are mineral salts needed for nerve and muscle function © Mc. Graw Hill 5
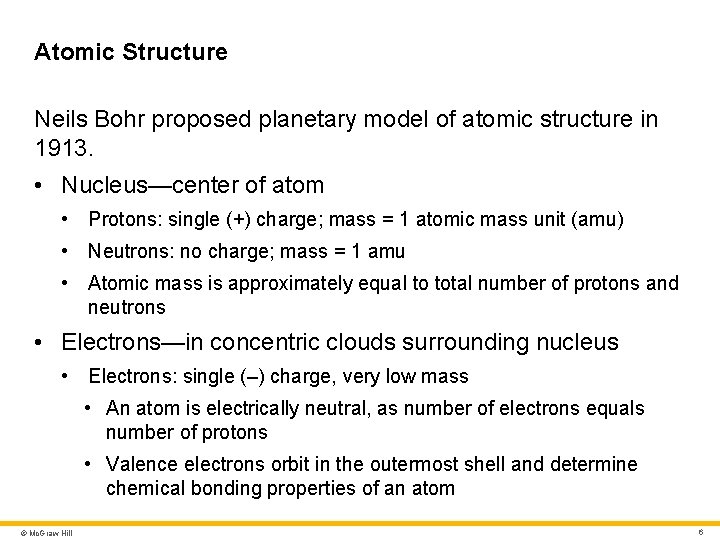
Atomic Structure Neils Bohr proposed planetary model of atomic structure in 1913. • Nucleus—center of atom • Protons: single (+) charge; mass = 1 atomic mass unit (amu) • Neutrons: no charge; mass = 1 amu • Atomic mass is approximately equal to total number of protons and neutrons • Electrons—in concentric clouds surrounding nucleus • Electrons: single (–) charge, very low mass • An atom is electrically neutral, as number of electrons equals number of protons • Valence electrons orbit in the outermost shell and determine chemical bonding properties of an atom © Mc. Graw Hill 6
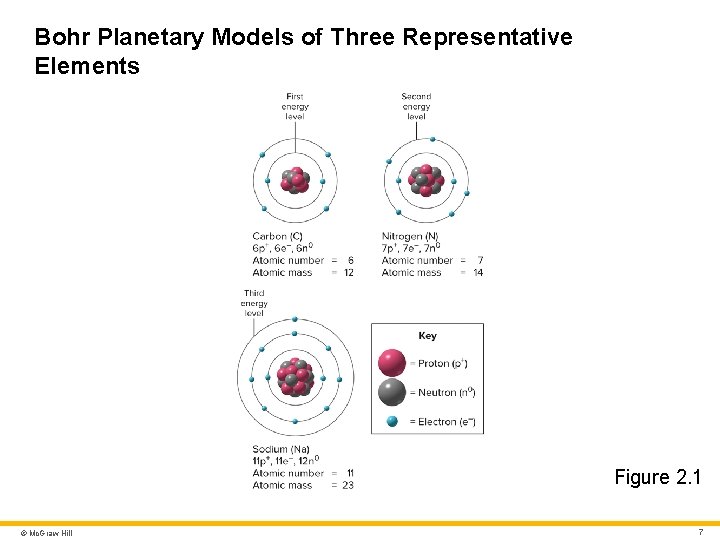
Bohr Planetary Models of Three Representative Elements Figure 2. 1 © Mc. Graw Hill 7
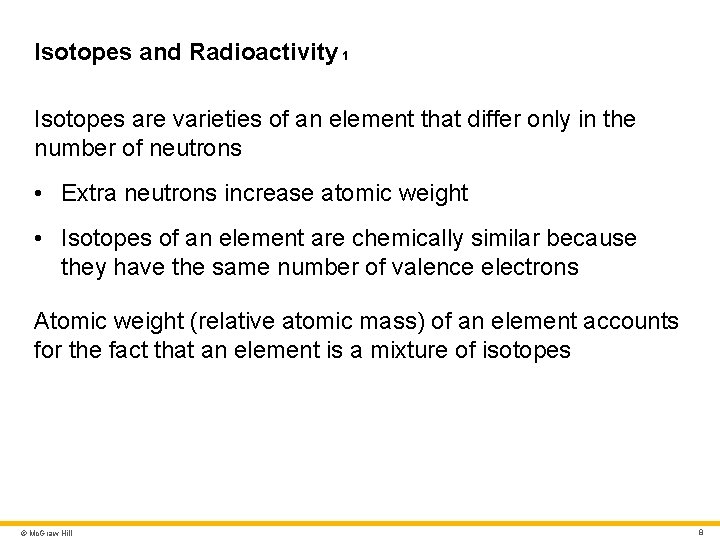
Isotopes and Radioactivity 1 Isotopes are varieties of an element that differ only in the number of neutrons • Extra neutrons increase atomic weight • Isotopes of an element are chemically similar because they have the same number of valence electrons Atomic weight (relative atomic mass) of an element accounts for the fact that an element is a mixture of isotopes © Mc. Graw Hill 8
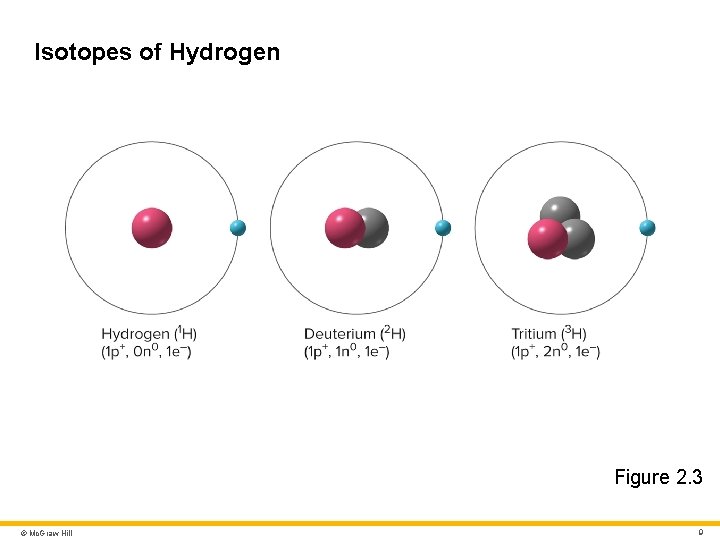
Isotopes of Hydrogen Figure 2. 3 © Mc. Graw Hill 9
Isotopes and Radioactivity 2 Radioisotopes • Unstable isotopes that decay and give off radiation • Every element has at least one radioisotope Intense radiation can be ionizing (ejects electrons, destroys molecules, creates free radicals) and can cause genetic mutations and cancer. • Examples: UV radiation, X-rays, alpha particles, beta particles, gamma rays Physical half-life of radioisotopes • Time required for 50% to decay to a stable state Biological half-life of radioisotopes • Time required for 50% to disappear from the body © Mc. Graw Hill 10
Isotopes and Radioactivity 3 Sievert (Sv)—unit of radiation dosage • 5 Sv or more is usually fatal • Standard acceptable exposure = 50 m. Sv per year Background radiation • Natural sources such as radon gas and cosmic rays • Average = 2. 4 m. Sv per year Artificial sources of radiation • X-rays, color TVs, and so on • Average = 0. 6 m. SV per year © Mc. Graw Hill 11

Radiation and Madame Curie First woman to receive Nobel Prize (1903) First woman in world to receive a Ph. D • Coined term radioactivity • Discovered radioactivity of polonium and radium • Trained physicians in use of X-rays and pioneered radiation therapy as cancer treatment Died of radiation poisoning at age 67 © Mc. Graw Hill Figure 2. 2 Source: Library of Congress Prints and Photographs Division 12

Ions, Electrolytes, and Free Radicals 1 Ion • Charged particle (atom or molecule) with unequal number of protons and electrons Ionization • Transfer of electrons from one atom to another Anion • Particle that has a net negative charge (due to gain of electrons) Cation • Particle that has a net positive charge (due to loss of electrons) Ions with opposite charges are attracted to each other © Mc. Graw Hill 13
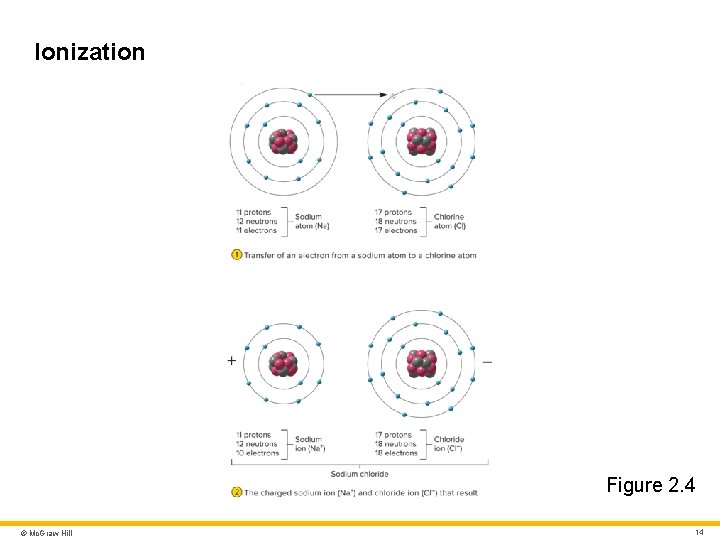
Ionization Figure 2. 4 © Mc. Graw Hill 14

Ions, Electrolytes, and Free Radicals 2 Electrolytes • Substances that ionize in water and form solutions capable of conducting electric current Electrolyte importance • Chemical reactivity, osmotic effects, electrical excitability of nerve and muscle • Electrolyte balance is one of the most important considerations in patient care (imbalances can lead to coma or cardiac arrest) © Mc. Graw Hill 15

Ions, Electrolytes, and Free Radicals 3 Free radicals • Short-lived particles with an unusual number of electrons • Produced by normal metabolic reactions, radiation, certain chemicals • Trigger reactions that destroy molecules, and can cause cancer, death of heart tissue, and aging Antioxidants • Chemicals that neutralize free radicals • Superoxide dismutase (SOD) is an antioxidant enzyme in the body • Selenium, vitamin E, vitamin C, and carotenoids are antioxidants obtained through the diet © Mc. Graw Hill 16
Molecules and Chemical Bonds 1 Molecule • Particle composed of two or more atoms united by a chemical bond Compound • Molecule composed of two or more different elements Molecular formula • Identifies constituent elements and how many atoms of each are present Structural formula • Identifies location of each atom Isomers • Molecules with identical molecular formulae but different arrangements of atoms © Mc. Graw Hill 17
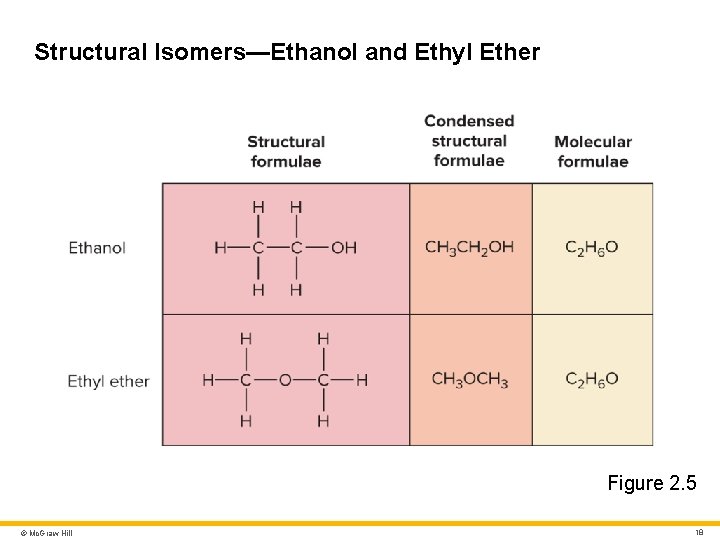
Structural Isomers—Ethanol and Ethyl Ether Figure 2. 5 © Mc. Graw Hill 18
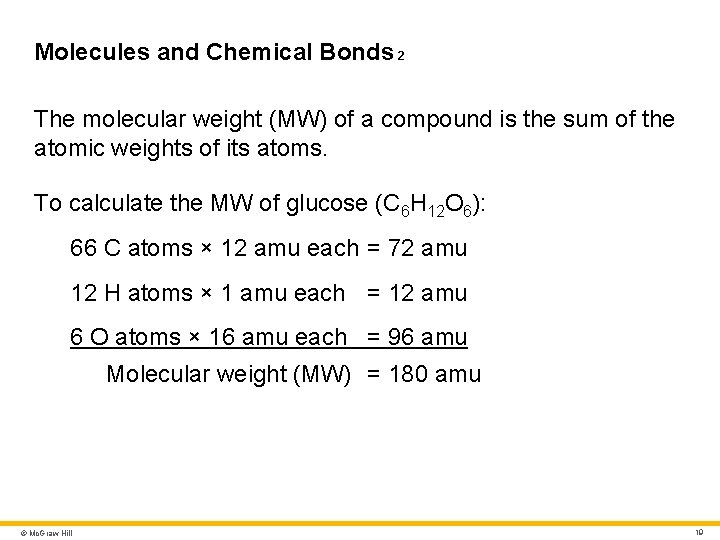
Molecules and Chemical Bonds 2 The molecular weight (MW) of a compound is the sum of the atomic weights of its atoms. To calculate the MW of glucose (C 6 H 12 O 6): 66 C atoms × 12 amu each = 72 amu 12 H atoms × 1 amu each = 12 amu 6 O atoms × 16 amu each = 96 amu Molecular weight (MW) = 180 amu © Mc. Graw Hill 19

Molecules and Chemical Bonds 3 Chemical bonds • Hold atoms together within a molecule, or attract one molecule to another • Important types include ionic bonds, covalent bonds, hydrogen bonds, and van der Walls forces Ionic bond • Attraction of a cation to an anion • Easily broken by water Covalent bond • Atoms share one or more pairs of electrons • Single covalent bond: nuclei share 1 pair of electrons • Double covalent bond: nuclei share 2 pairs of electrons © Mc. Graw Hill 20
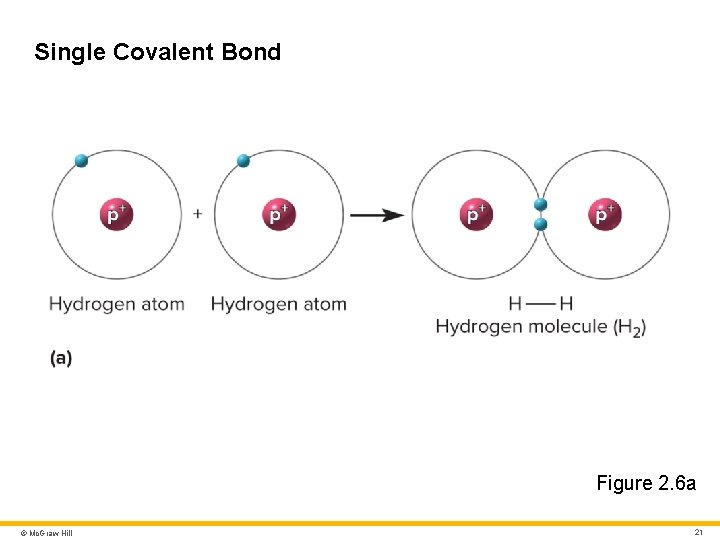
Single Covalent Bond Figure 2. 6 a © Mc. Graw Hill 21
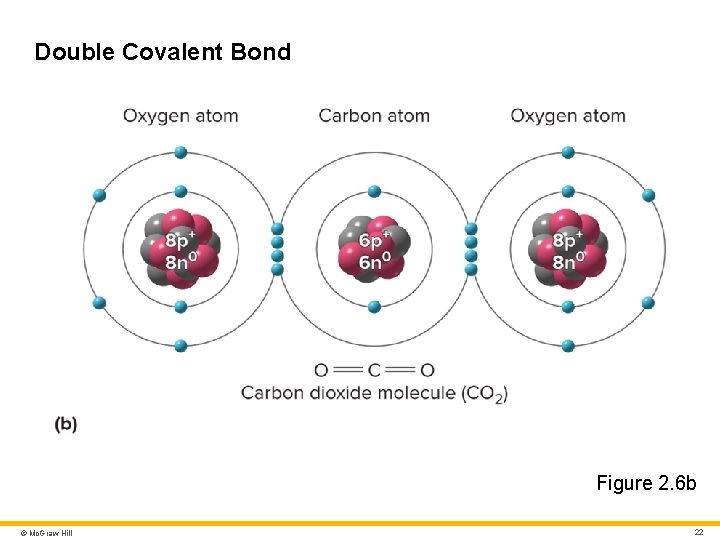
Double Covalent Bond Figure 2. 6 b © Mc. Graw Hill 22
Molecules and Chemical Bonds 4 Covalent bond (continued) • Nonpolar covalent bond: electrons shared equally • Polar covalent bond: electrons shared unequally (spend more time near oxygen) © Mc. Graw Hill 23
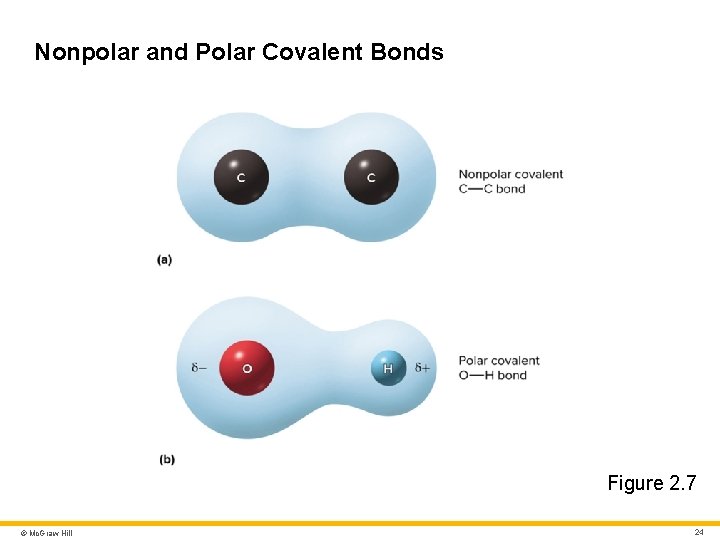
Nonpolar and Polar Covalent Bonds Figure 2. 7 © Mc. Graw Hill 24
Molecules and Chemical Bonds 5 Hydrogen bond • Weak attraction between a slightly positive hydrogen atom in one molecule and a slightly negative oxygen or nitrogen atom in another atom • Important to physiology • Water molecules are attracted to each other by hydrogen bonds. • Large molecules (DNA and proteins) are shaped in part by the formation of hydrogen bonds within them. © Mc. Graw Hill 25
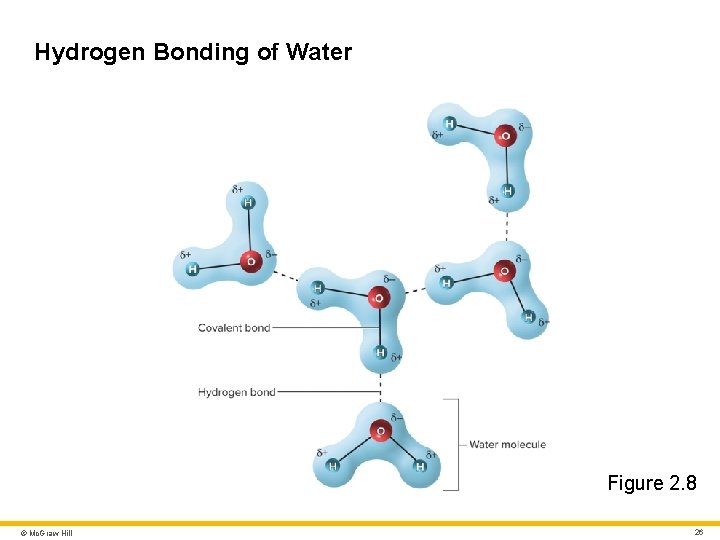
Hydrogen Bonding of Water Figure 2. 8 © Mc. Graw Hill 26

Molecules and Chemical Bonds 6 Van der Waals forces • Weak, brief attractions between neutral atoms • Fluctuation in electron density within an atom creates polarity for a moment, and attracts adjacent atom for a very short time • Only 1% as strong as a covalent bond, but play important role in physiology (e. g. , protein folding) © Mc. Graw Hill 27

2. 2 Water and Mixtures Expected Learning Outcomes: • Define mixture and distinguish between mixtures and compounds. • Describe the biologically important properties of water. • Show three kinds of mixtures differ from each other. • Define acid and base and interpret the p. H scale. • Discuss some ways in which the concentration of a solution can be expressed, and the kinds of information we can derive from the different units of measure. © Mc. Graw Hill 28
Water and Mixtures • Consist of substances that are physically blended but not chemically combined • Body fluids are complex mixtures of chemicals Water • Most mixtures in our bodies consist of chemicals dissolved or suspended in water • Water is 50– 75% of body weight • Depends on age, sex, fat content, and so on © Mc. Graw Hill 29

Water 1 Polar covalent bonds and a V-shaped molecule give water a set of properties that account for its ability to support life • Solvency • Cohesion • Adhesion • Chemical reactivity • Thermal stability © Mc. Graw Hill 30

Water 2 Solvency • Ability to dissolve other chemicals • Water is called the universal solvent • Metabolic reactions depend on solvency of water Hydrophilic • Substances that dissolve in water • Hydrophilic molecules are polarized or charged (e. g. , sugar) Hydrophobic • Substances that do not dissolve in water • Hydrophobic molecules are nonpolar or neutral (e. g. , fats) © Mc. Graw Hill 31

Water 3 Attractions to water molecules overpower the ionic bond in Na. Cl • Water forms hydration spheres around each ion and the salt dissolves • Water’s negative pole faces Na+, its positive pole faces Cl– © Mc. Graw Hill 32
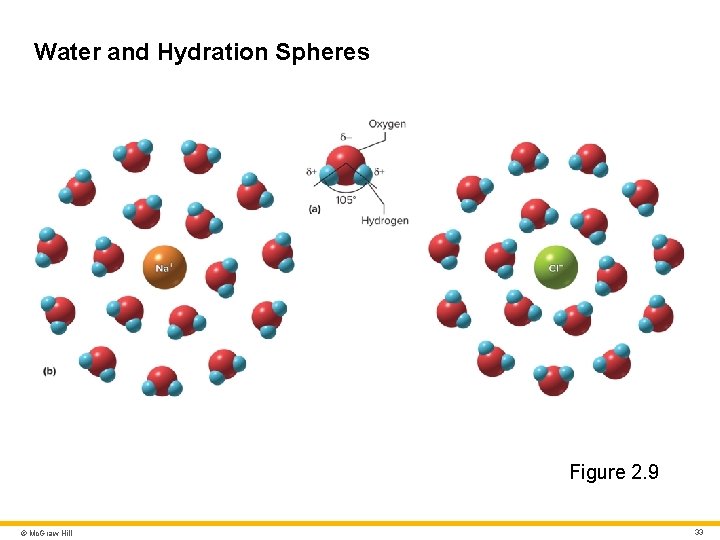
Water and Hydration Spheres Figure 2. 9 © Mc. Graw Hill 33

Water 4 Adhesion • Tendency of one substance to cling to another • Water adheres to large membranes reducing friction around organs Cohesion • Tendency of like molecules to cling to each other • Water is very cohesive due to its hydrogen bonds • Surface film on surface of water is due to molecules being held together by surface tension © Mc. Graw Hill 34

Water 5 Chemical reactivity • Ability to participate in chemical reactions • Water ionizes into H+ and OH– • Water ionizes many other chemicals (acids and salts) • Water is involved in hydrolysis and dehydration synthesis reactions © Mc. Graw Hill 35
Water 6 Heat capacity • Amount of heat needed to raise the temperature of 1 g of a substance by 1 °C Calorie (cal) • Base unit of heat • Amount of heat that raises the temperature of 1 g of water 1 °C Water’s thermal stability helps stabilize the internal temperature of the body • Water has high heat capacity • Hydrogen bonds resist temperature increases by inhibiting molecular motion • Water is an effective coolant • 1 ml of perspiration removes 500 calories © Mc. Graw Hill 36

Solutions, Colloids, and Suspensions 1 Solution • Consists of particles called the solute mixed with a more abundant substance (usually water) called the solvent • Solute can be gas, solid, or liquid • Solutions are defined by the following properties: • Solute particles under 1 nm • Solute particles do not scatter light • Will pass through most membranes • Will not separate on standing © Mc. Graw Hill 37
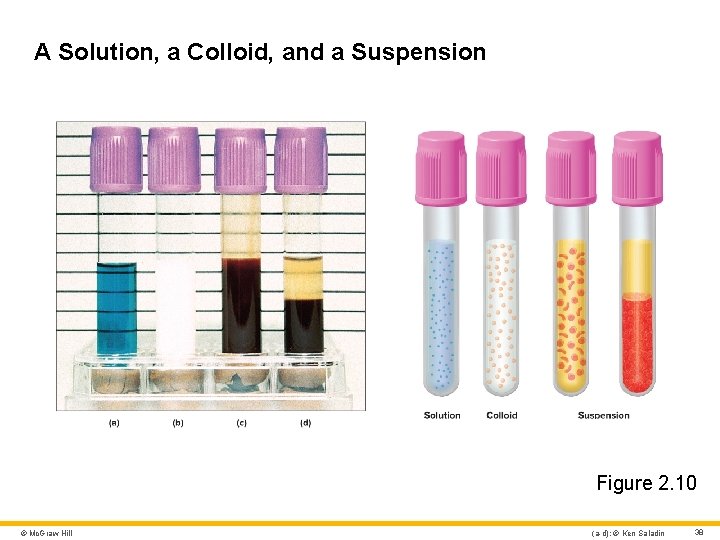
A Solution, a Colloid, and a Suspension Figure 2. 10 © Mc. Graw Hill (a-d): © Ken Saladin 38
Solutions, Colloids, and Suspensions 2 Colloids • Colloids in the body are often mixtures of protein and water • Many can change from liquid to gel state within and between cells • Colloids are defined by the following physical properties: • Particles range from 1– 100 nm in size • Scatter light and are usually cloudy • Particles too large to pass through semipermeable membrane • Particles remain permanently mixed with the solvent when mixture stands © Mc. Graw Hill 39

Solutions, Colloids, and Suspensions 3 Suspension • Defined by the following physical properties: • Particles exceed 100 nm • Too large to penetrate selectively permeable membranes • Cloudy or opaque in appearance • Separates on standing • Example: blood cells in blood plasma Emulsion • Suspension of one liquid in another • Examples: oil-and-vinegar salad dressing; fat in breast milk © Mc. Graw Hill 40
Acids, Bases, and p. H 1 Acid • Proton donor (releases H+ ions in water) Base • Proton acceptor (accepts H+ ions or releases OH− ions) p. H • Measure of acidity derived from the molarity of H+ • p. H of 7. 0 is neutral (H+ = OH−) • p. H of less than 7 is acidic (H+ > OH−) • p. H of greater than 7 is basic (OH− > H+) • Maintaining normal (slightly basic) p. H of blood is crucial for physiological functions • Buffers are chemical solutions that resist changes in p. H © Mc. Graw Hill 41
Acids, Bases, and p. H 2 p. H is the negative logarithm of hydrogen ion molarity • ph = –log[H+] • Example: if [H+] = 10– 3, then p. H = –log[10– 3] = 3 A change of one number on the p. H scale represents a tenfold change in H+ concentration • p. H 4. 0 is 10 times as acidic as p. H 5. 0 © Mc. Graw Hill 42
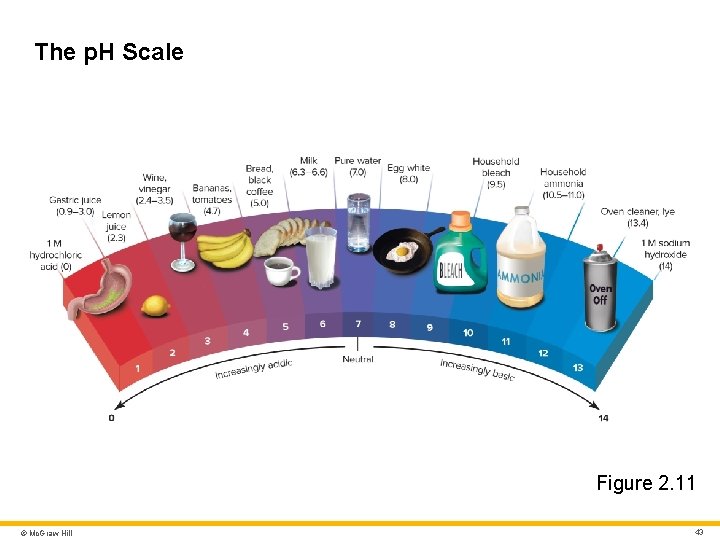
The p. H Scale Figure 2. 11 © Mc. Graw Hill 43
Other Measures of Concentration 1 How much solute is in a given volume of solution? • Weight per volume • Weight of solute in a given volume of solution • Example: IV saline contains 8. 5 g Na. Cl per liter of solution. • Common biology units: milligrams per deciliter (mg/dl) • Example: Serum cholesterol may be 200 mg/dl • Percentages • Might be weight of solute (solid) per volume • Example: 5% dextrose solution has 5 g solute in 100 ml solution. • Might be volume of solute (liquid) per volume of solution • Example: 70% ethanol has 70 ml of ethanol in 100 ml solution. © Mc. Graw Hill 44
Other Measures of Concentration 2 Electrolytes are crucial for heart, nerve, and muscle Electrolyte concentration is measured in equivalents (Eq) • 1 Eq = amount of electrolyte needed to neutralize 1 mole of H+ or OH– ions • Often expressed as milliequivalents (m. Eq/L) • To calculate, multiply molar concentration and valence of ion • 1 m. M Na+ = 1 m. Eq/L • 1 m. M Na 2+ = 2 m. Eq/L © Mc. Graw Hill 45
2. 3 Energy and Chemical Reactions Expected Learning Outcomes • Define energy and work, and describe some types of energy. • Understand how chemical reactions are symbolized by chemical equations. • List and define the fundamental types of chemical reactions. • Identify the factors that govern the speed and direction of a reaction. • Define metabolism and its two subdivisions. • Define oxidation and reduction, and relate these to changes in the energy content of a molecule. © Mc. Graw Hill 46

Energy and Work 1 Energy • Capacity to do work • To do work means to move something • All body activities are forms of work Potential energy • Energy stored in an object, but not currently doing work • Example: water behind a dam Chemical energy • Potential energy in molecular bonds Free energy • Potential energy available in a system to do useful work © Mc. Graw Hill 47
Energy and Work 2 Kinetic energy • Energy of motion • Doing work • Example: water flowing through a dam, generating electricity Heat • Kinetic energy of molecular motion Electromagnetic energy • Kinetic energy of moving packets of radiation called photons © Mc. Graw Hill 48
Classes of Chemical Reactions 1 Chemical reaction • Process in which a covalent or ionic bond is formed or broken Chemical equation • Symbolizes the course of a chemical reaction • Reactants (on left) → products (on right) Classes of chemical reactions include: • Decomposition reactions • Synthesis reactions • Exchange reactions © Mc. Graw Hill 49
Classes of Chemical Reactions 2 Decomposition reactions • Large molecule breaks down into two or more smaller ones • AB → A + B Synthesis reactions • Two or more small molecules combine to form a larger one • A + B → AB Exchange reactions • Two molecules exchange atoms or group of atoms • AB + CD → ABCD → AC + BD • Example: stomach acid (HCl) and sodium bicarbonate (Na. HCO 3) from the pancreas combine to form Na. Cl and H 2 CO 3 © Mc. Graw Hill 50
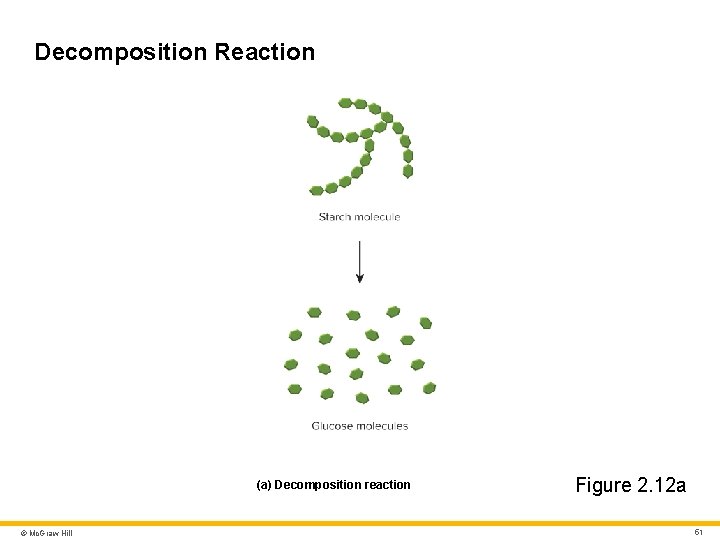
Decomposition Reaction (a) Decomposition reaction © Mc. Graw Hill Figure 2. 12 a 51
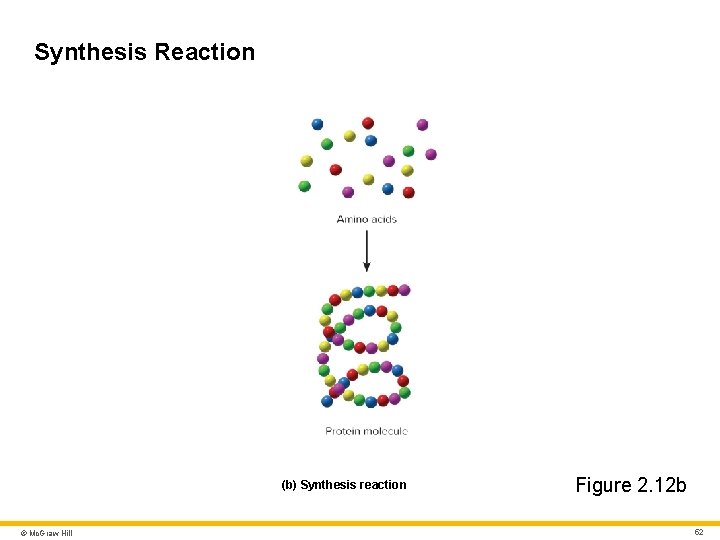
Synthesis Reaction (b) Synthesis reaction © Mc. Graw Hill Figure 2. 12 b 52
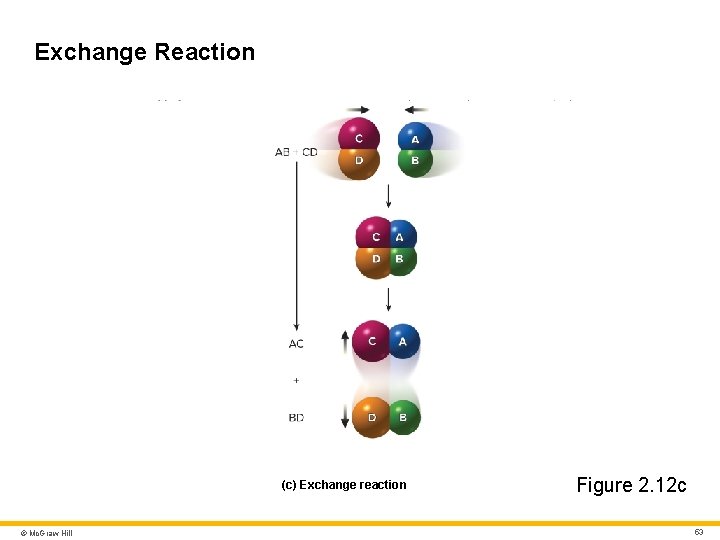
Exchange Reaction (c) Exchange reaction © Mc. Graw Hill Figure 2. 12 c 53
Classes of Chemical Reactions 3 Reversible reactions • Can go in either direction under different circumstances • Symbolized with double-headed arrow • Example: CO 2 + H 2 O ↔ H 2 CO 3 ↔ HCO 3− + H+ • An important reaction in respiratory, urinary, and digestive physiology Law of mass action • Direction of reaction determined by relative abundance of substances on either side of equation • Equilibrium is reached when ratio of products to reactants is stable © Mc. Graw Hill 54
Reaction Rates Reactions occur when molecules collide with enough force and correct orientation Reaction rates increase when: • Concentration of reactants increases • Temperature rises • A catalyst is present • Enzyme catalysts bind to reactants and hold them in orientations that facilitate the reaction • Catalysts are not changed by the reaction and can repeat the process frequently © Mc. Graw Hill 55
Metabolism, Oxidation, and Reduction 1 Metabolism • All chemical reactions of the body Catabolism • Energy-releasing (exergonic) decomposition reactions • Breaks covalent bonds • Produces smaller molecules Anabolism • Energy-storing (endergonic) synthesis reactions • Requires energy input • Example: production of protein or fat Catabolism and anabolism are inseparably linked • Anabolism is driven by energy released by catabolism. © Mc. Graw Hill 56
Metabolism, Oxidation, and Reduction 2 Oxidation • A chemical reaction in which a molecule gives up electrons and releases energy • Molecule is oxidized when it loses electrons • The oxidizing agent is the electron acceptor • Oxygen is often the electron acceptor (oxidizing agent) Reduction • Any chemical reaction in which a molecule gains electrons and energy • Molecule is reduced when it accepts electrons • The reducing agent is the molecule that donates electrons © Mc. Graw Hill 57
Metabolism, Oxidation, and Reduction 3 Oxidation-reduction (redox) reactions • Oxidation of one molecule is always accompanied by reduction of another • Electrons are often transferred as hydrogen atoms © Mc. Graw Hill 58
2. 4 Organic Compounds Expected Learning Outcomes: • Explain why carbon is especially well suited to serve as the structural foundation of many biological molecules. • Identify some common functional groups of organic molecules from their formulae. • Discuss the relevance of polymers to biology and explain how they are formed and broken by dehydration synthesis and hydrolysis. • Discuss the types and functions of carbohydrates, lipids, and proteins. • Explain how enzymes function. • Describe the structure, production, and function of ATP. • Identify other nucleotide types and their functions and the principal types of nucleic acids. © Mc. Graw Hill 59
Carbon Compounds and Functional Groups 1 Organic chemistry • The study of compounds containing carbon Four categories of carbon compounds: • Carbohydrates • Lipids • Proteins • Nucleic acids © Mc. Graw Hill 60

Carbon Compounds and Functional Groups 2 Carbon has four valence electrons • Can form four covalent bonds with other atoms Carbon atoms bind readily with each other to form carbon backbones • Form long chains, branched molecules, and rings • Readily bond with hydrogen, oxygen, nitrogen, sulfur, and other elements Functional groups • Small clusters of atoms attached to carbon backbone • Determine many of the properties of organic molecules • Examples: hydroxyl, methyl, carboxyl, amino, phosphate © Mc. Graw Hill 61
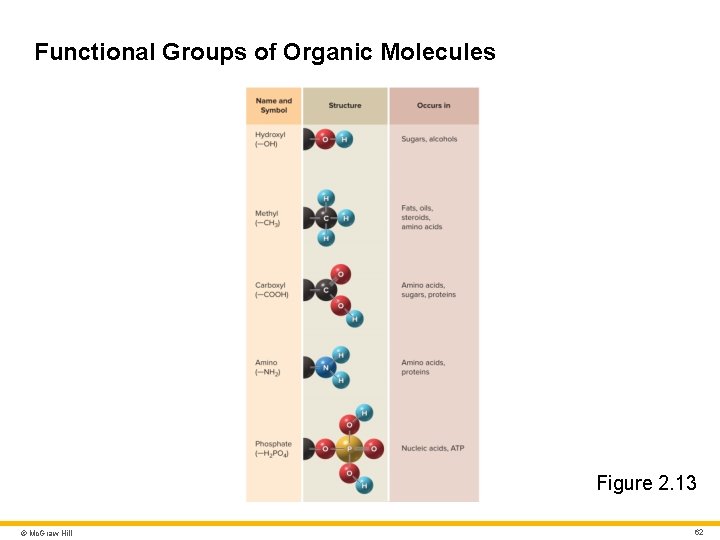
Functional Groups of Organic Molecules Figure 2. 13 © Mc. Graw Hill 62
Monomers and Polymers 1 Macromolecules • Very large organic molecules with high molecular weights Polymers • Macromolecules made of a repetitive series of identical or similar subunits (monomers) • Example: starch is a polymer of about 3, 000 glucose monomers Polymerization • Joining monomers to form a polymer © Mc. Graw Hill 63
Monomers and Polymers 2 Dehydration synthesis (condensation) • Monomers covalently bind together to form a polymer • A hydroxyl (-OH) group is removed from one monomer, and a hydrogen (-H) from another • Water produced as a by-product Hydrolysis • The opposite of dehydration synthesis • Splitting a polymer in monomers by the addition of water • Enzyme helps break the covalent bond that links two monomers together • A water molecule ionizes into OH- and H+ • OH- is added to one monomer • H+ is added to the other monomer © Mc. Graw Hill 64
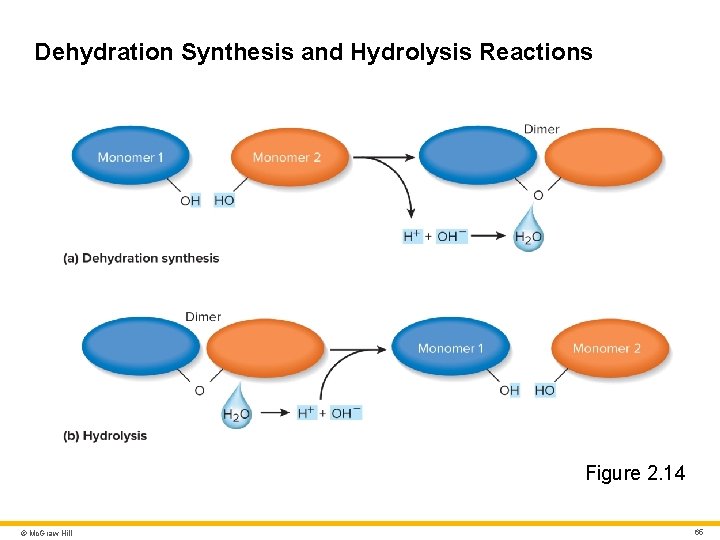
Dehydration Synthesis and Hydrolysis Reactions Figure 2. 14 © Mc. Graw Hill 65
Carbohydrates 1 Carbohydrates are hydrophilic organic molecules • Examples: sugars and starches • 2: 1 ratio of hydrogen to oxygen • General formula: (CH 2 O)n, n = number of carbon atoms • Glucose, n = 6, so formula is C 6 H 12 O 6 Names of carbohydrates often built from the root “sacchar-” and the suffix “-ose” both meaning sugar, sweet © Mc. Graw Hill 66
Carbohydrates 2 Monosaccharides • Simplest carbohydrates • Monomers Three important monomers are glucose, galactose, and fructose • Produced by digestion of more complex carbohydrates • Glucose is blood sugar • All three have the same molecular formula: C 6 H 12 O 6 • Isomers of each other © Mc. Graw Hill 67
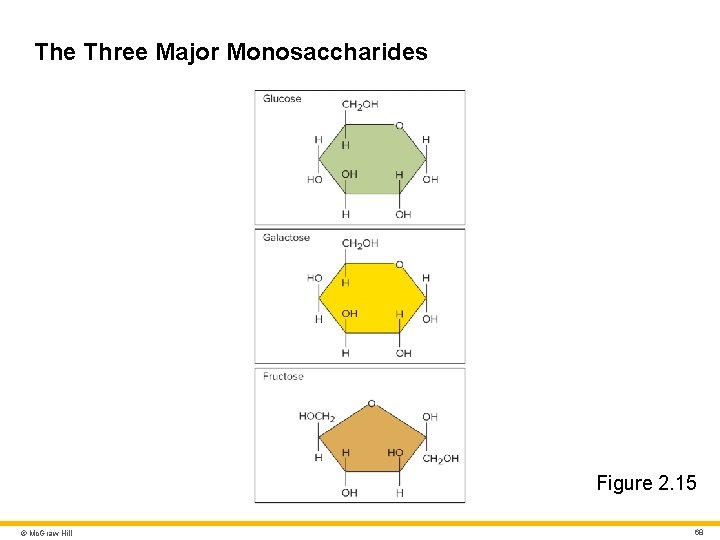
The Three Major Monosaccharides Figure 2. 15 © Mc. Graw Hill 68

Carbohydrates 3 Disaccharides • Sugars made of two covalently bonded monosaccharides Three important disaccharides: • Sucrose (table sugar) • Glucose + fructose • Lactose (milk sugar) • Glucose + galactose • Maltose (sugar in grain products) • Glucose + glucose © Mc. Graw Hill 69
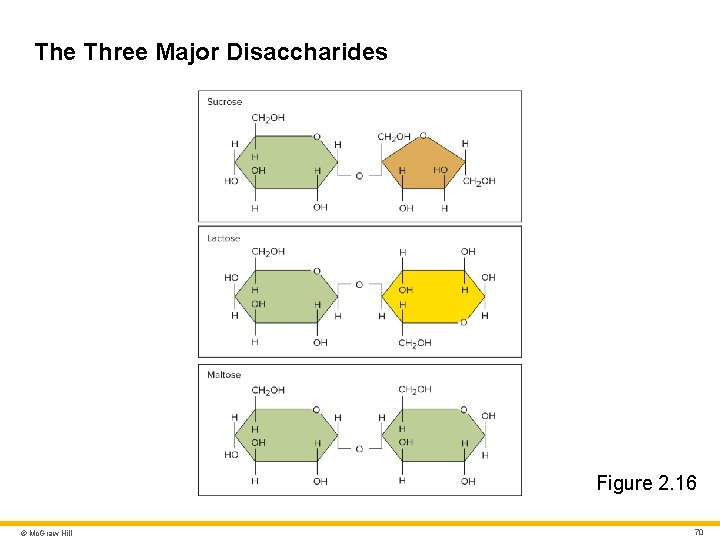
The Three Major Disaccharides Figure 2. 16 © Mc. Graw Hill 70

Carbohydrates 4 Oligosaccharides • Short chains of three or more monosaccharides (at least 10) Polysaccharides • Long chains of monosaccharides (at least 50) Three important polysaccharides: • Glycogen • Energy storage in cells of liver, muscle, brain, uterus, vagina • Starch • Energy storage in plants that is digestible by humans • Cellulose • Structural molecule in plants that is important for human dietary fiber (but indigestible to us) © Mc. Graw Hill 71
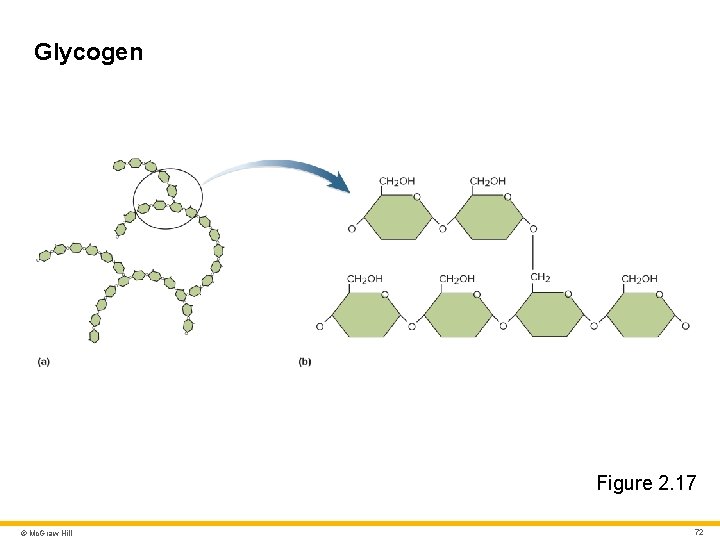
Glycogen Figure 2. 17 © Mc. Graw Hill 72
Carbohydrates 5 Carbohydrates are a quickly mobilized source of energy • All digested carbohydrates converted to glucose • Oxidized to make ATP Carbohydrates are often conjugated with lipids or proteins • Lipid and protein molecules at the external surface of the cell membrane often have chains of sugars attached to them • Glycolipids • Glycoproteins are also a major component of mucus • Proteoglycans are more carbohydrate than protein • Gels that hold cells and tissues together; fill umbilical cord and eye • Joint lubrication; responsible for the rubbery texture of cartilage © Mc. Graw Hill 73

Lipids 1 Lipids are hydrophobic organic molecules with a high ratio of hydrogen to oxygen • More calories per gram than carbohydrates • Five primary types of lipids in the human body: • Fatty acids • Triglycerides • Phospholipids • Eicosanoids • Steroids © Mc. Graw Hill 74

Lipids 2 Fatty acids • Chains of 4– 24 carbon atoms with carboxyl group on one end and methyl group on the other • Essential fatty acids must be obtained from food • Fatty acids are classified as saturated or unsaturated • Saturated fatty acids • Carbon atoms linked by single covalent bonds • Molecule contains as much hydrogen as possible (“saturated” with hydrogen) • Unsaturated fatty acids • Contain some double bonds between carbons • Molecule has potential to add hydrogen • Polyunsaturated fatty acids have multiple double bonds between carbons © Mc. Graw Hill 75

Lipids 3 Triglycerides (Neutral Fats) • Three fatty acids linked to glycerol • Formed by dehydration synthesis; broken down by hydrolysis • Primary function is energy storage • Also help with insulation and shock absorption (adipose tissue) Dietary oils and fats are triglycerides • “Oils” are usually liquid at room or body temperature • Example: plant-derived polyunsaturated triglycerides such as corn and olive oils • “Fats” are usually solid at room or body temperature • Example: animal-derived saturated triglycerides (e. g. , animal fat) • The difference between “oil” and “fat” is somewhat arbitrary • Example: coconut oil is solid at room temperature © Mc. Graw Hill 76
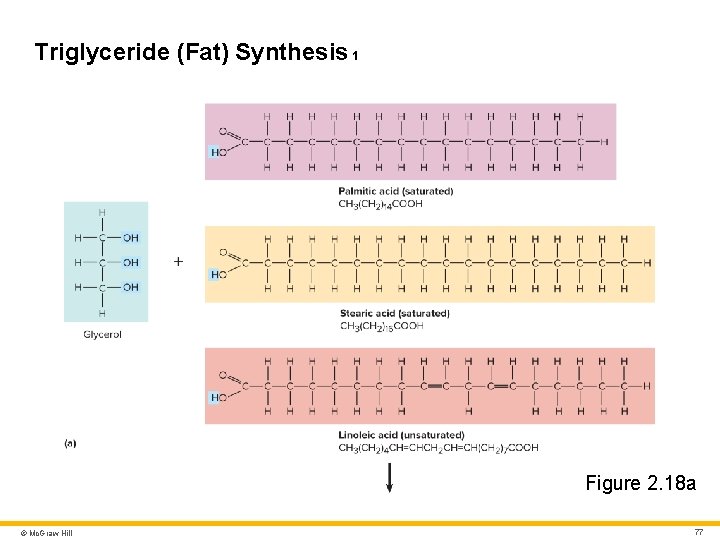
Triglyceride (Fat) Synthesis 1 Figure 2. 18 a © Mc. Graw Hill 77
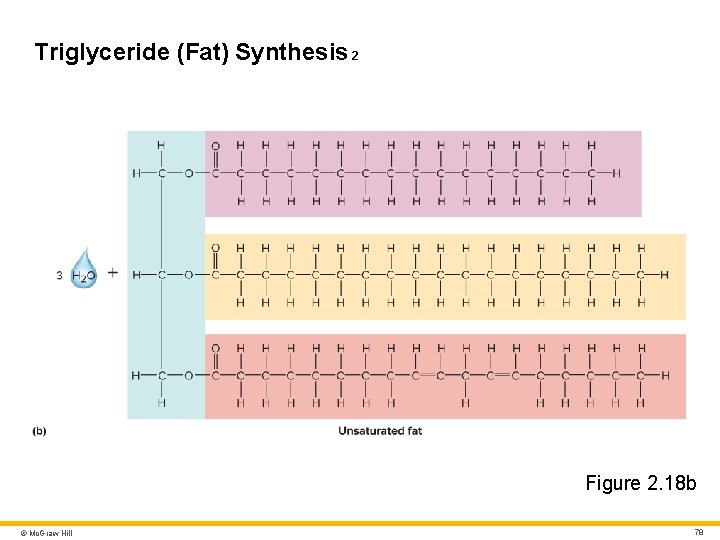
Triglyceride (Fat) Synthesis 2 Figure 2. 18 b © Mc. Graw Hill 78
Trans Fats and Cardiovascular Health Trans-fatty acids • Two covalent single C – C bonds angle in opposites (trans, “across from each other”) on each side of the C = C double bond • Resist enzymatic breakdown in the human body, remain in circulation longer, deposits in the arteries; thus, raises the risk of heart disease Cis-fatty acids • Two covalent single C – C bonds angle in the same direction adjacent to the C = C double bond © Mc. Graw Hill 79
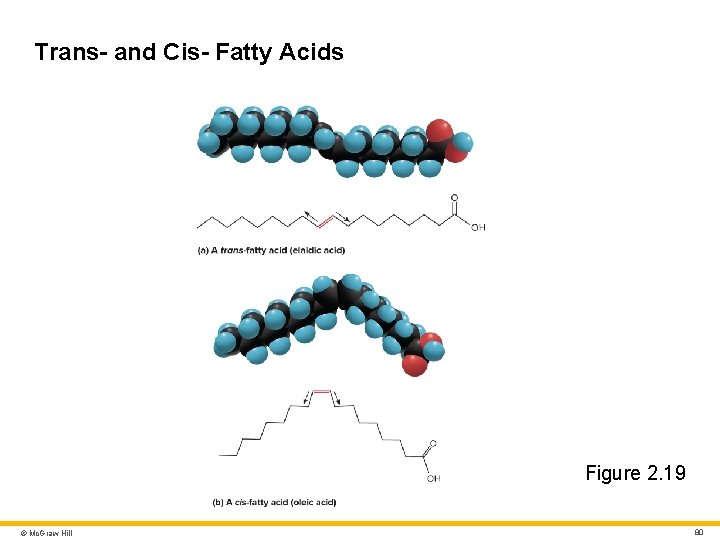
Trans- and Cis- Fatty Acids Figure 2. 19 © Mc. Graw Hill 80

Lipids 4 Phospholipids • Similar to neutral fats except one fatty acid is replaced by a phosphate group • Structural foundation of cell membrane • Amphipathic • Fatty acid “tails” are hydrophobic • Phosphate “head” is hydrophilic © Mc. Graw Hill 81
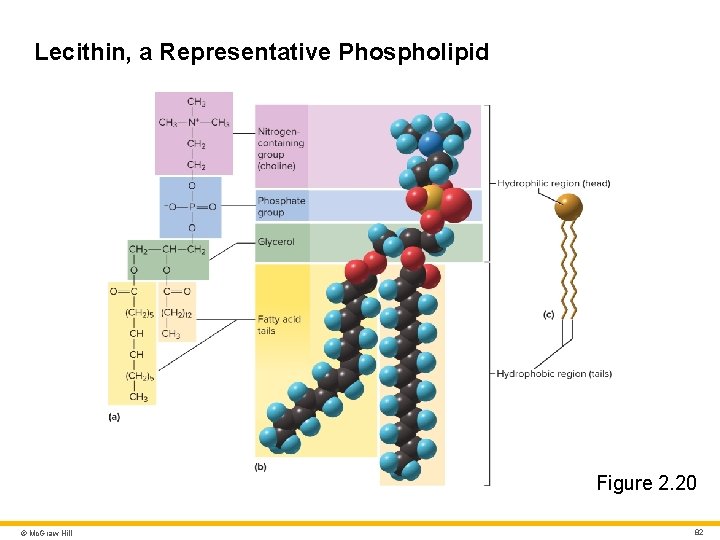
Lecithin, a Representative Phospholipid Figure 2. 20 © Mc. Graw Hill 82
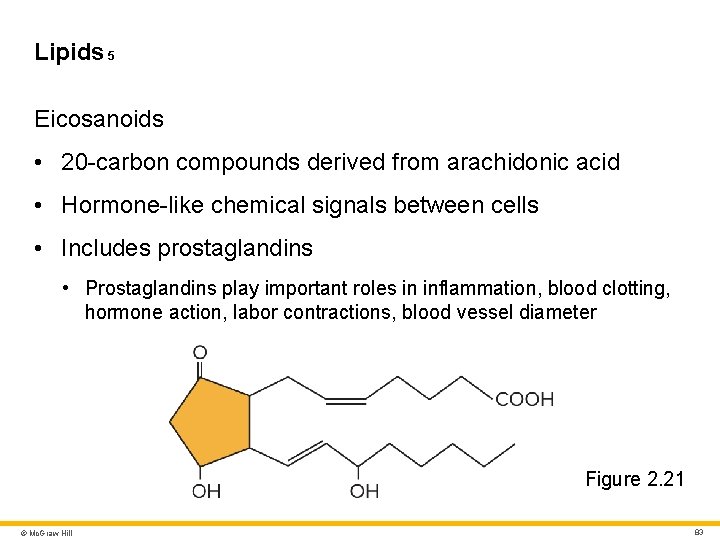
Lipids 5 Eicosanoids • 20 -carbon compounds derived from arachidonic acid • Hormone-like chemical signals between cells • Includes prostaglandins • Prostaglandins play important roles in inflammation, blood clotting, hormone action, labor contractions, blood vessel diameter Figure 2. 21 © Mc. Graw Hill 83

Lipids 6 Steroid • Lipid with 17 carbon atoms in four rings Cholesterol • The “parent” steroid from which other steroids are synthesized • Important for nervous system function and structural integrity of all cell membranes • 15% of our cholesterol comes from diet • 85% is internally synthesized (mostly in liver) Other steroids include cortisol, progesterone, estrogens, testosterone, and bile acids © Mc. Graw Hill 84
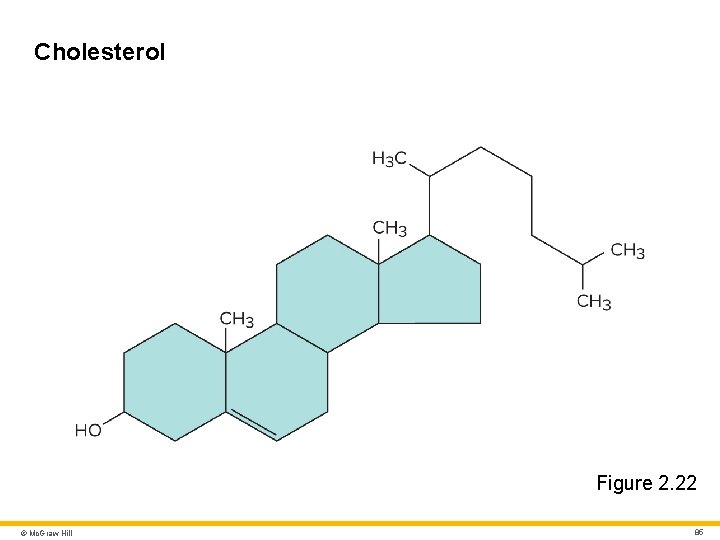
Cholesterol Figure 2. 22 © Mc. Graw Hill 85

“Good” and “Bad” Cholesterol There is only one kind of cholesterol “Good” and “bad” cholesterol refer to droplets of lipoprotein in the blood that are complexes of cholesterol, fat, phospholipid, and protein • HDL (high-density lipoprotein) = “good cholesterol” • Lower ratio of lipid to protein • May help to prevent cardiovascular disease • LDL (low-density lipoprotein) = “bad cholesterol” • High ratio of lipid to protein • Contributes to cardiovascular disease © Mc. Graw Hill 86
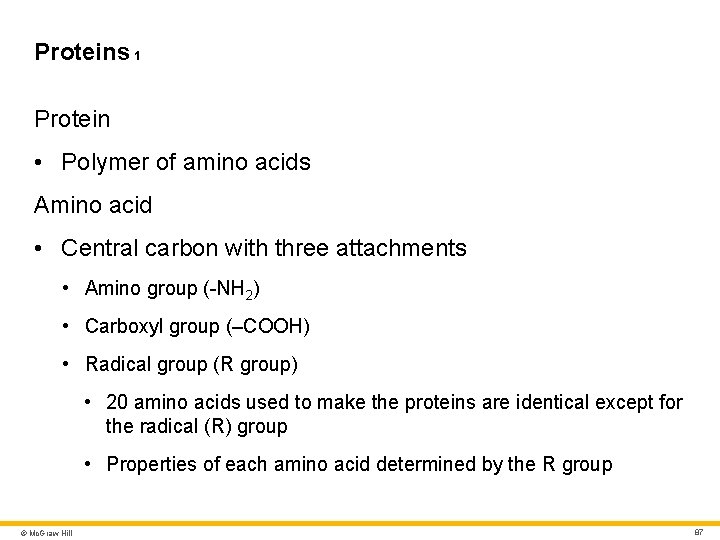
Proteins 1 Protein • Polymer of amino acids Amino acid • Central carbon with three attachments • Amino group (-NH 2) • Carboxyl group (–COOH) • Radical group (R group) • 20 amino acids used to make the proteins are identical except for the radical (R) group • Properties of each amino acid determined by the R group © Mc. Graw Hill 87
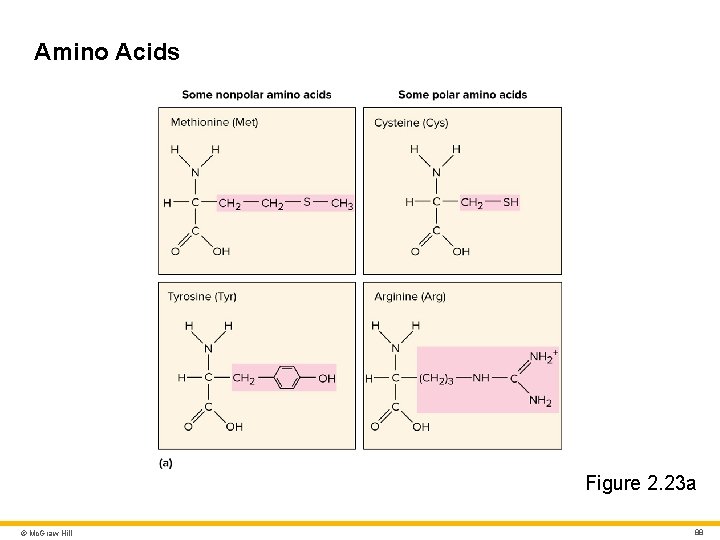
Amino Acids Figure 2. 23 a © Mc. Graw Hill 88

Proteins 2 Peptide • Composed of two or more amino acids joined by peptide bonds Peptide bond • Joins the amino group of one amino acid to the carboxyl group of the next • Formed by dehydration synthesis Peptides are named for the number of amino acids they contain • Dipeptides (2 amino acids) • Tripeptides (3 amino acids) • Oligopeptides (between 3 and 15 amino acids) • Polypeptides (between 15 and 50 amino acids) • Proteins (more than 50 amino acids) © Mc. Graw Hill 89
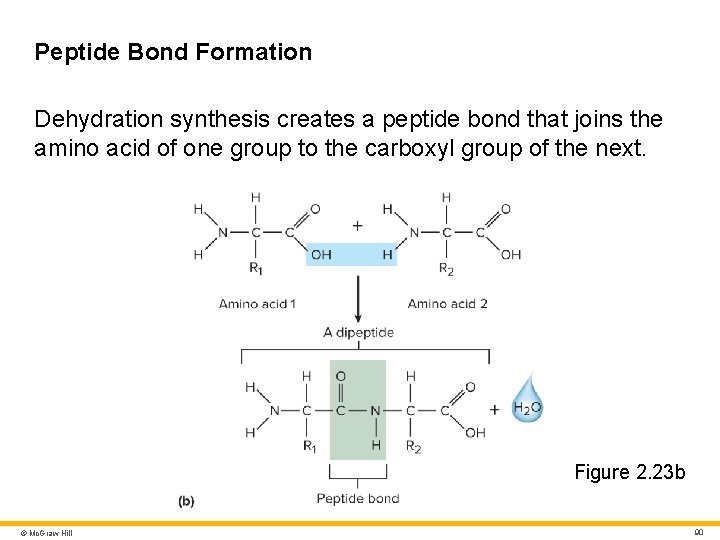
Peptide Bond Formation Dehydration synthesis creates a peptide bond that joins the amino acid of one group to the carboxyl group of the next. Figure 2. 23 b © Mc. Graw Hill 90
Protein Structure 1 Conformation • Unique, three-dimensional shape of protein crucial to function • Proteins can reversibly change conformation to affect function • Important examples seen in muscle contraction, enzyme catalysis, membrane channel opening, and so on Denaturation • Extreme conformational change that destroys function • Extreme heat or p. H may cause permanent (irreversible) denaturation • Example: cooked egg white becomes opaque and stiff © Mc. Graw Hill 91
Protein Structure 2 Primary structure • Sequence of amino acids within protein molecule • Primary structure is encoded by genes Secondary structure • Coiled or folded shape held together by hydrogen bonds • Hydrogen bonds between slightly negative C = O and slightly positive N – H groups • Most common secondary structures are: • Alpha helix (spring-like shape) • Beta-pleated sheet (folded, ribbon-like shape) © Mc. Graw Hill 92
Protein Structure 3 Tertiary structure • Further bending and folding of proteins into globular and fibrous shapes due to hydrophobic–hydrophilic interactions and van der Waals forces • Globular proteins • Compact tertiary structure for proteins within cell membrane and proteins that move freely in body fluids • Fibrous proteins • Slender filaments suited for roles in muscle contraction and strengthening of skin and hair © Mc. Graw Hill 93
Protein Structure 4 Quaternary structure • Associations of two or more polypeptide chains due to ionic bonds and hydrophobic–hydrophilic interactions • Occurs only in some proteins • Example: hemoglobin has four peptide subunits © Mc. Graw Hill 94
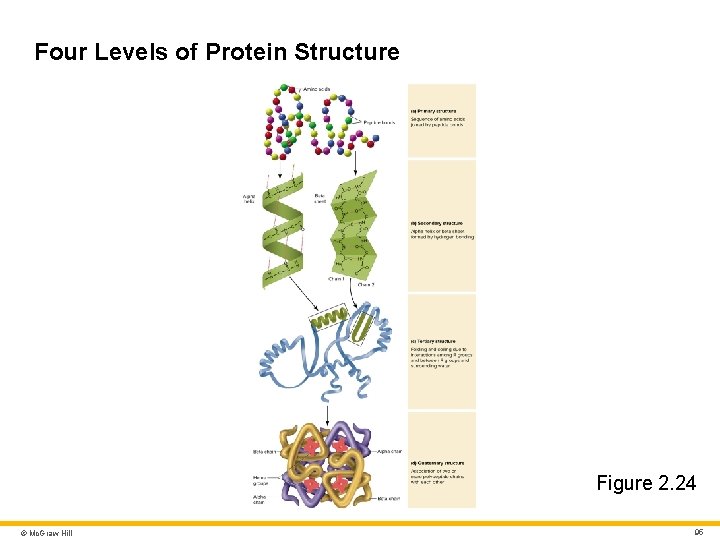
Four Levels of Protein Structure Figure 2. 24 © Mc. Graw Hill 95
Protein Structure 5 Conjugated proteins contain a non-amino acid moiety called a prosthetic group covalently bound to them • Example: hemoglobin contains four complex ironcontaining rings called a heme moiety (see previous slide) © Mc. Graw Hill 96
Protein Functions 1 Structure • Keratin • Tough structural protein of hair, nails, skin surface • Collagen • Contained in deeper layers of skin, bones, cartilage, and teeth Communication • Neurotransmitters, some hormones, and other signaling molecules are proteins • Signaling molecules that exert their effects by reversibly binding to a receptor molecule are called ligands • The receptors to which the signaling molecules bind are also proteins © Mc. Graw Hill 97
Protein Functions 2 Membrane transport • Channel proteins allow hydrophilic substances to diffuse across cell membranes • Carrier proteins help solutes cross cell membranes via active or passive transport Catalysis • The enzymes that catalyze physiological reactions are usually globular proteins Recognition and protection • Glycoproteins are important for immune recognition • Antibodies are proteins © Mc. Graw Hill 98

Protein Functions 3 Movement • Motor proteins are molecules with the ability to change shape repeatedly Cell adhesion • Proteins bind cells together © Mc. Graw Hill 99
Enzymes and Metabolism Enzymes • Proteins that function as biological catalysts • Lower activation energy (the energy needed to get a reaction started) • Permit reactions to occur rapidly at body temperature Substrate • Substance an enzyme acts upon Enzyme naming convention • Named for substrate with -ase as the suffix • Examples: amylase catalyzes the hydrolysis of amylose (starch); lactase catalyzes the hydrolysis of lactose (milk sugar) © Mc. Graw Hill 100
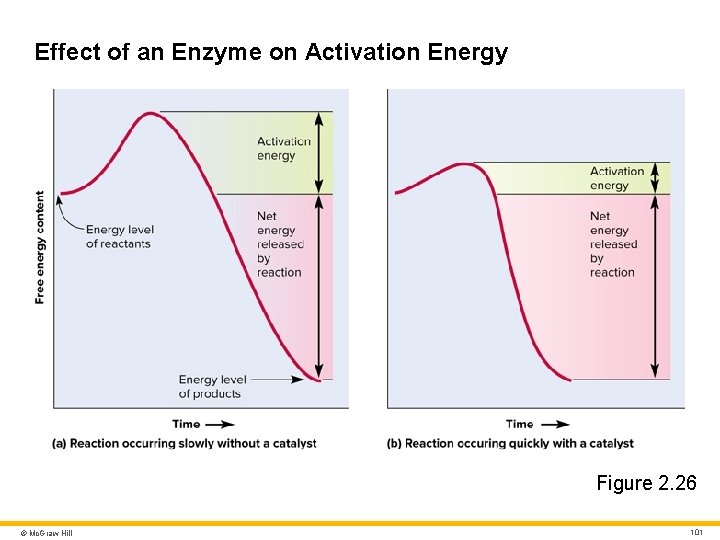
Effect of an Enzyme on Activation Energy Figure 2. 26 © Mc. Graw Hill 101

Enzyme Structure and Action 1 Enzyme action • Substrate binds to enzyme’s active site • Molecules form enzyme–substrate complex • Enzyme–substrate specificity (lock and key) • Enzyme releases reaction products • Enzyme unchanged and can repeat process • Example: sucrose hydrolysis © Mc. Graw Hill 102
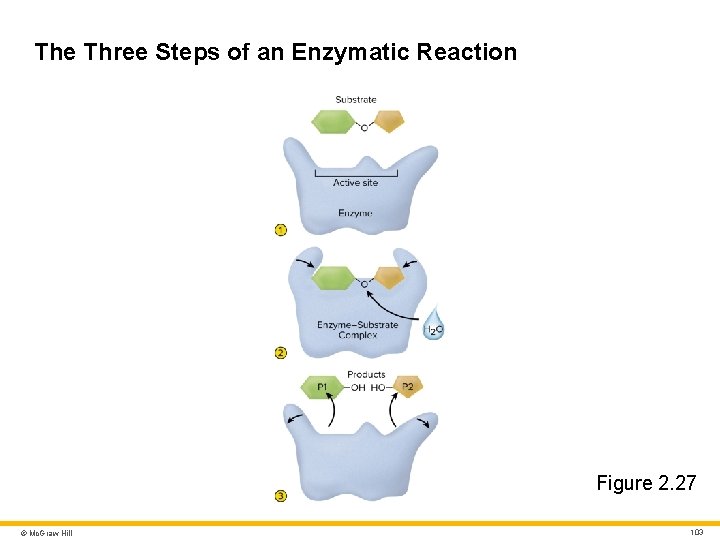
The Three Steps of an Enzymatic Reaction Figure 2. 27 © Mc. Graw Hill 103
Enzyme Structure and Action 2 Reusability of enzymes • Enzymes are not consumed by the reactions Astonishing speed • One enzyme molecule can catalyze millions of reactions per minute Temperature, p. H and other factors can change enzyme shape and function • Can alter ability of enzyme to bind to substrate • Enzymes vary in optimum p. H • Salivary amylase works best at p. H 7. 0 • Pepsin in stomach works best at p. H 2. 0 • Temperature optimum for human enzymes is usually near body temperature (37°C) © Mc. Graw Hill 104

Cofactors • Non-protein “helper” molecules whose presence is necessary for many enzymes to function • About two-thirds of human enzymes require a cofactor • Some cofactors are inorganic ions such as iron, copper, zinc, magnesium, and calcium • Some cofactors work by binding to the enzyme and triggering a conformational change that activates the active site Coenzymes • Organic cofactors • Often derived from vitamins • Example: NAD+ • Derived from niacin • Plays important role in metabolism and ATP production by shuttling electrons between enzymes in respiration pathways © Mc. Graw Hill 105
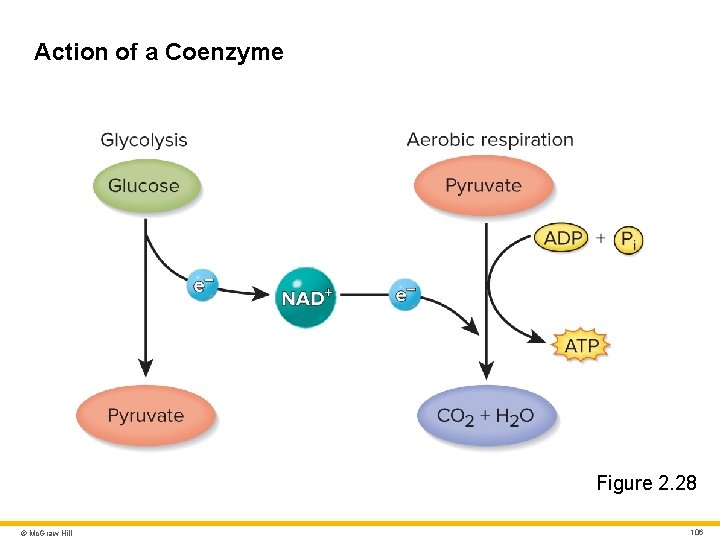
Action of a Coenzyme Figure 2. 28 © Mc. Graw Hill 106
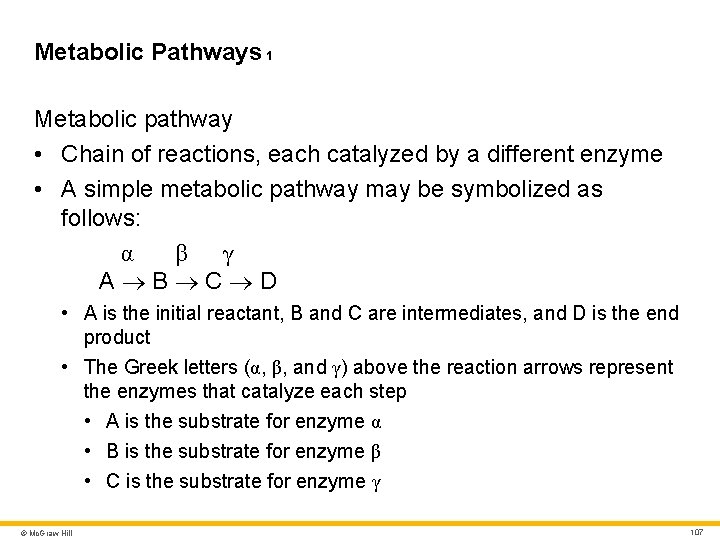
Metabolic Pathways 1 Metabolic pathway • Chain of reactions, each catalyzed by a different enzyme • A simple metabolic pathway may be symbolized as follows: α β γ A B C D • A is the initial reactant, B and C are intermediates, and D is the end product • The Greek letters (α, β, and γ) above the reaction arrows represent the enzymes that catalyze each step • A is the substrate for enzyme α • B is the substrate for enzyme β • C is the substrate for enzyme γ © Mc. Graw Hill 107
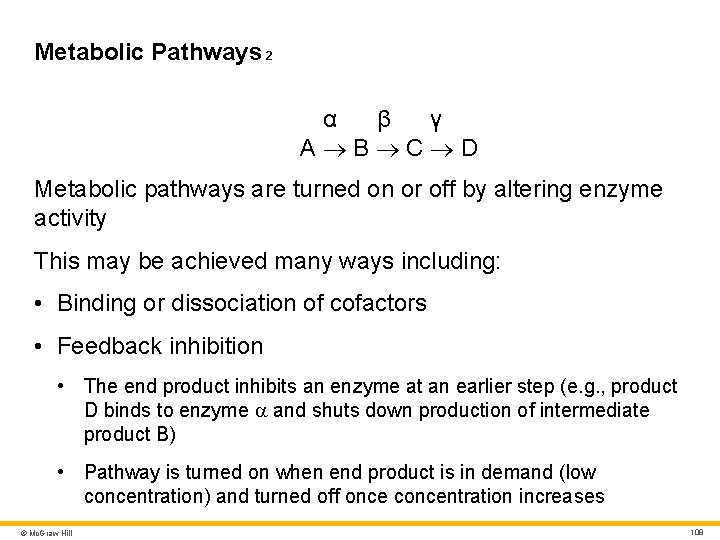
Metabolic Pathways 2 α β γ A B C D Metabolic pathways are turned on or off by altering enzyme activity This may be achieved many ways including: • Binding or dissociation of cofactors • Feedback inhibition • The end product inhibits an enzyme at an earlier step (e. g. , product D binds to enzyme a and shuts down production of intermediate product B) • Pathway is turned on when end product is in demand (low concentration) and turned off once concentration increases © Mc. Graw Hill 108
ATP, Other Nucleotides, and Nucleic Acids Nucleotides • Organic compounds with three principal components: • Nitrogenous base (single or double carbon–nitrogen ring) • Sugar (monosaccharide) • One or more phosphate groups • Examples of nucleotides: • ATP (Adenosine triphosphate) • c. AMP (Cyclic adenosine monophosphate) © Mc. Graw Hill 109
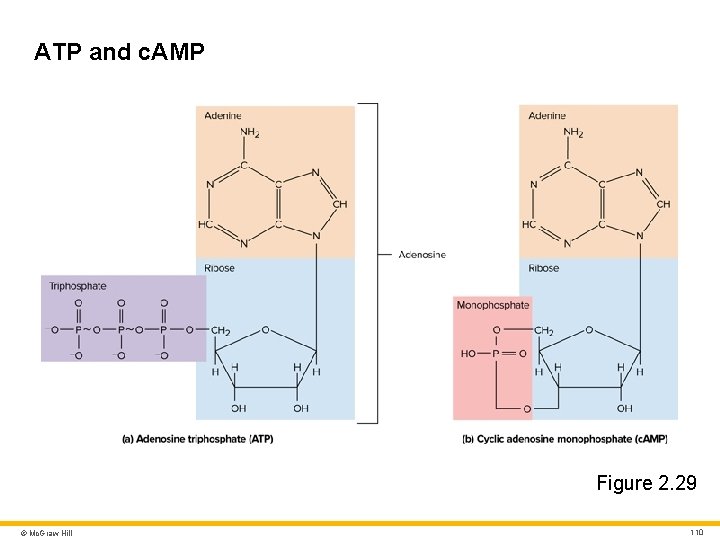
ATP and c. AMP Figure 2. 29 © Mc. Graw Hill 110
Adenosine Triphosphate 1 ATP is the body’s most important energy-transfer molecule • Stores energy gained from exergonic reactions • Releases it within seconds for physiological work • Holds energy in covalent bonds • Second and third phosphate groups have high energy bonds (~) • Most energy transfers to and from ATP involve adding or removing the third phosphate group © Mc. Graw Hill 111
Adenosine Triphosphate 2 Hydrolysis of ATP is catalyzed by adenosine triphosphatases (ATPases) • Breaks the third high-energy phosphate bond • Separates ATP into ADP + Pi + energy Phosphorylation • Addition of free phosphate group to a molecule • Carried out by enzymes called kinases © Mc. Graw Hill 112
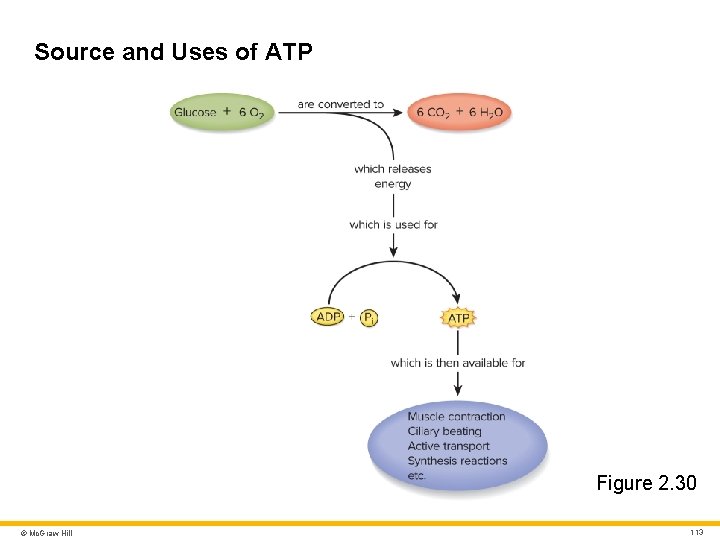
Source and Uses of ATP Figure 2. 30 © Mc. Graw Hill 113

ATP Production 1 Glucose oxidation and ATP synthesis • Glycolysis: splitting glucose into two pyruvates • If ATP demand outpaces oxygen supply pyruvate anaerobically ferments to lactate • If enough oxygen present aerobic respiration occurs in mitochondria © Mc. Graw Hill 114
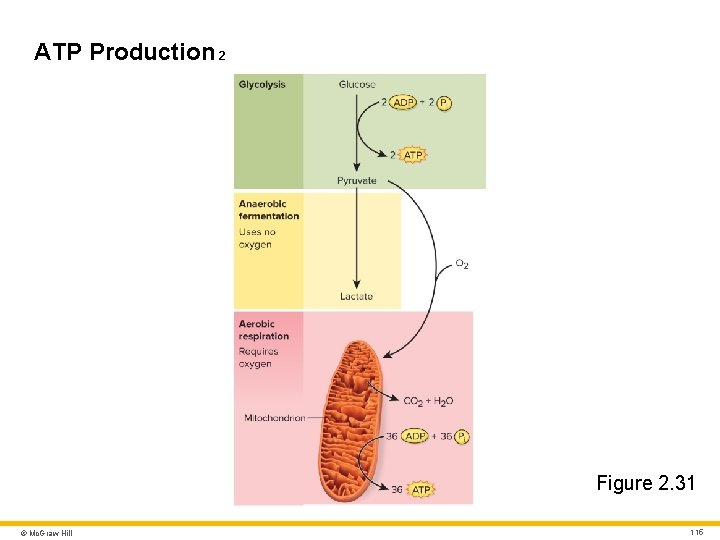
ATP Production 2 Figure 2. 31 © Mc. Graw Hill 115
Other Nucleotides Guanosine triphosphate (GTP) • Another nucleotide involved in energy transfer Cyclic adenosine monophosphate (c. AMP) • Formed by removal of second and third phosphate groups from ATP • Formation triggered by hormone binding to cell surface • c. AMP becomes “second messenger” within cell © Mc. Graw Hill 116
Nucleic Acids Nucleic acids are polymers of nucleotides DNA (deoxyribonucleic acid) • Contains millions of nucleotides • Constitutes genes • Instructions for synthesizing proteins RNA (ribonucleic acid) • Three types: messenger RNA (m. RNA), ribosomal RNA (r. RNA), and transfer RNA (t. RNA) • 70 to 10, 000 nucleotides long • Carries out genetic instruction for synthesizing proteins • Assembles amino acids in right order to produce proteins © Mc. Graw Hill 117

Because learning changes everything. www. mheducation. com © 2021 Mc. Graw Hill. All rights reserved. Authorized only for instructor use in the classroom. No reproduction or further distribution permitted without the prior written consent of Mc. Graw Hill. ®
- Slides: 118