Battery Current Amps Volts Watts Insulator Conductor Electric





• • • Battery Current Amps Volts Watts Insulator Conductor Electric motors Generators • • Shocked Superconductor Resistor Charge Ohm Switch Circuit House Wires
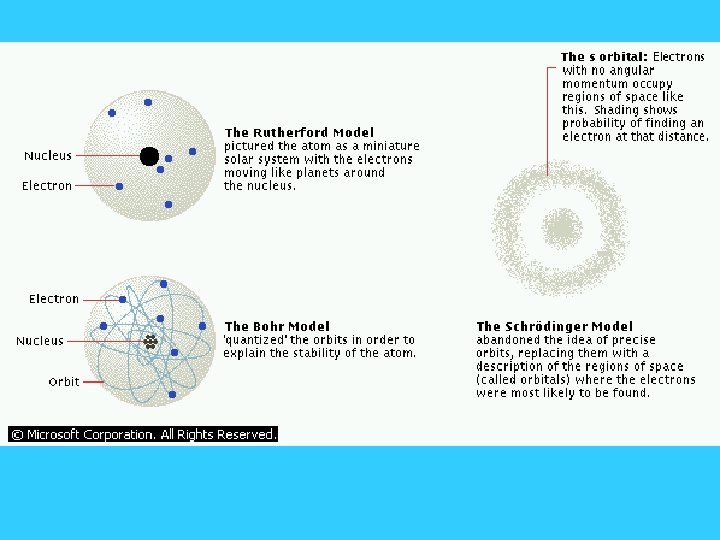
Brief Review – Structure of the Atom • Atoms are composed of several subatomic particles – electrons – protons – neutrons • A typical atom consists of a cloud of electrons surrounding the nucleus
Brief Review – Structure of the Atom • The electron is the fundamental negatively ( -) charged particle. Orbit the nucleus. – electrons have a very small mass compared to that of a proton • The proton is the fundamental positively charged (+) particle. Found in nucleus. – protons have a very large mass compared to the electron
Brief Review – Structure of the Atom • Neutrons – are neutral. They have no charge. Found in the nucleus. About same mass as proton. • Because atoms contain equal numbers of protons and electrons, they are electrically neutral.

Charging Objects • Because they are large and in the nucleus, protons and neutrons can not be removed from atoms by ordinary means. • Because of this, electrically charged objects are usually formed when neutral objects gain or lose electrons. • Electrons, however, can be removed from an atom when energy is imparted to the atom …….
1 st Rule • Only electrons move. – A negative charge is an excess of electrons. – A positive charge is a deficiency of electrons.

Physics Humor Two atoms are walking down the street. Says one atom to the other, "Hey! I think I lost an electron!” The other says, "Are you sure? ? " "Yes, I'm positive!"

“The Tale of the Tape” Quick Activity

2 nd Rule • From the tape demo, we can conclude that two objects with the same sign of charge (both + or both - ) are repelled by an electrical force, and objects of opposite sign of charge are attracted to each other. • Opposite charges attract; like charges repel

Third Rule • Balloon Demo – What happens when we turn the balloon around? – Why?
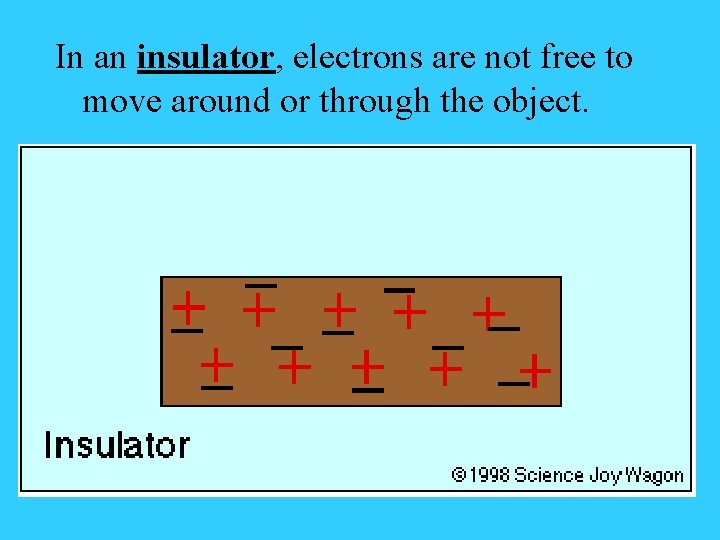
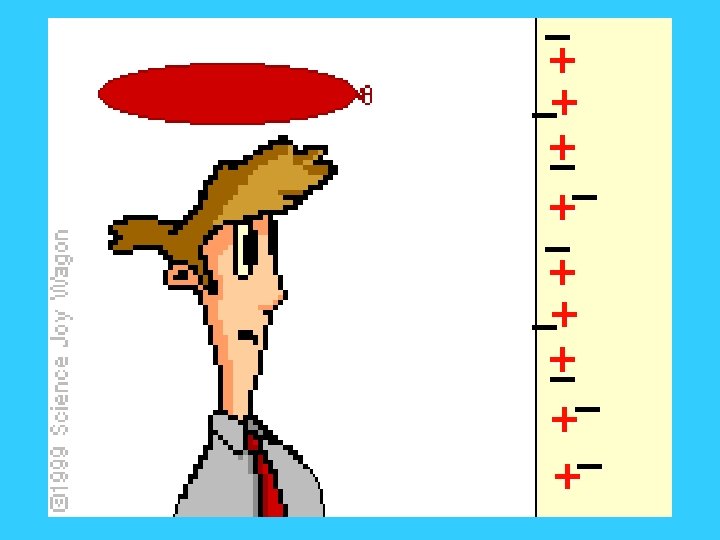
In an insulator, electrons are not free to move around or through the object.
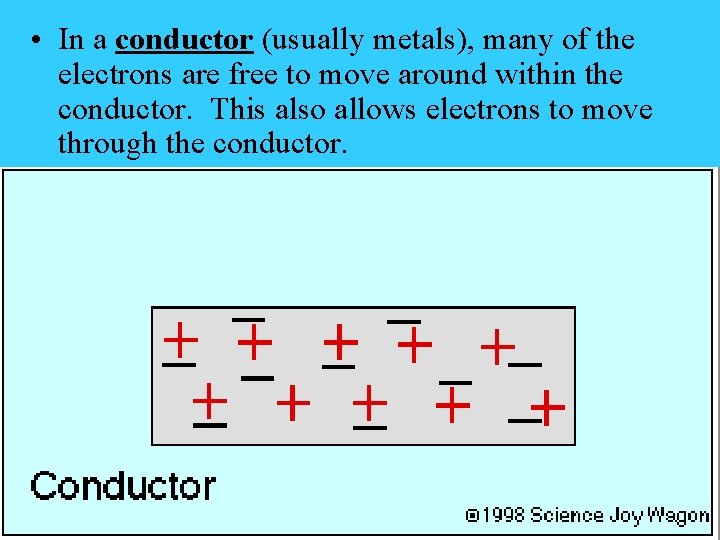
• In a conductor (usually metals), many of the electrons are free to move around within the conductor. This also allows electrons to move through the conductor.
Third Rule • Electrons can move freely through a conductor. Electrons can’t move through an insulator.
The Three Rules - Electrostatics 1. Electrons are the elementary charge that moves. 2. Opposite charges attract. Like charges repel. 3. Electrons move through conductors. Electrons don’t move through insulators.

Challenge Question • The balloon stole electrons from my hair when I rubbed it on my hair, so it became negative and my hair became positive…. • But the wall was neutral, and didn’t participate in any exchange of electrons… • So… why did the balloon stick to a neutral object?

Induction • The redistribution of charge within an object when a charged object is brought nearby. – The object remains neutral, but some of the charges separate. – When the charged object is removed, the original distribution of charge returns.

Activity • Electroscopes Lab • Charging by conduction – Charge left behind • Charging by induction – Charge left behind
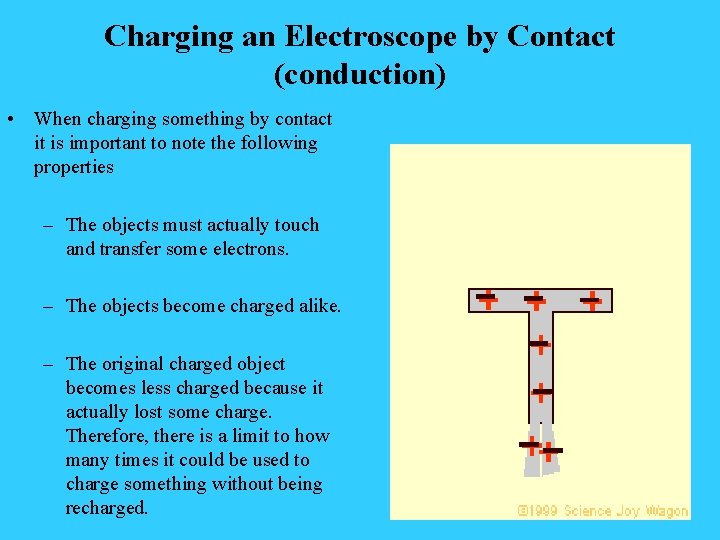
Charging an Electroscope by Contact (conduction) • When charging something by contact it is important to note the following properties – The objects must actually touch and transfer some electrons. – The objects become charged alike. – The original charged object becomes less charged because it actually lost some charge. Therefore, there is a limit to how many times it could be used to charge something without being recharged.
Charging by Electrostatic Induction • There are several advantages to charging something by induction. – The originally charged object never loses any charge so it need not be recharged. (work does not need to be done creating the charge again) – The induced charge can be quite strong and subsequent charges will be equally strong – Electroscope ends up oppositely charged to the object used to charge it.

Will a charged object such as a balloon, comb, or electroscope stay charged forever (as long as nothing touches it)?
Charge Theft

How do we know what steals electrons and what loses electrons? • Creating Charges with Friction

The comb and paper • If a system has only neutral objects, it has a total net charge of zero. • The comb and hair both started neutral.

The comb and paper • Rubbing the comb in hair transferred electrons to the comb, but this did not change the overall charge on the system – the system as a whole remains neutral. The hair is positively charged equal in magnitude to the negative charge on the comb.

Law of Conservation of Charge • In a closed, isolated system, the total charge of the system remains constant. • Charges within the system may be transferred from one object to another, but charge is neither created nor destroyed.
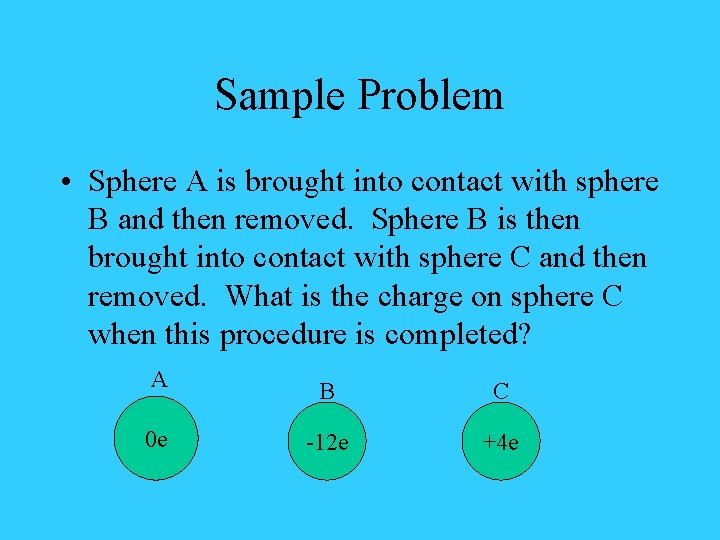
Sample Problem • Sphere A is brought into contact with sphere B and then removed. Sphere B is then brought into contact with sphere C and then removed. What is the charge on sphere C when this procedure is completed? A B C 0 e -12 e +4 e
Quantity of Charge • The SI unit of charge is the coulomb, C. • One coulomb is equal to 6. 25 x 1018 elementary charges. • The elementary charge, e, is equal in magnitude to the charge on an electron (-e) or the charge on a proton (+e).
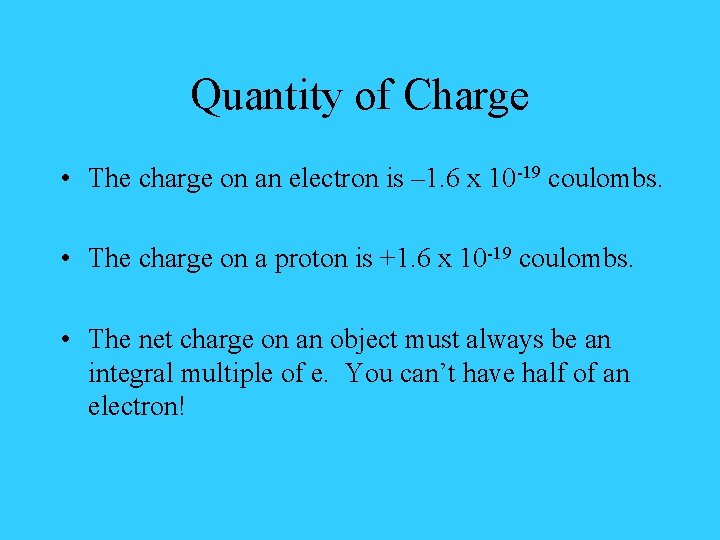
Quantity of Charge • The charge on an electron is – 1. 6 x 10 -19 coulombs. • The charge on a proton is +1. 6 x 10 -19 coulombs. • The net charge on an object must always be an integral multiple of e. You can’t have half of an electron!
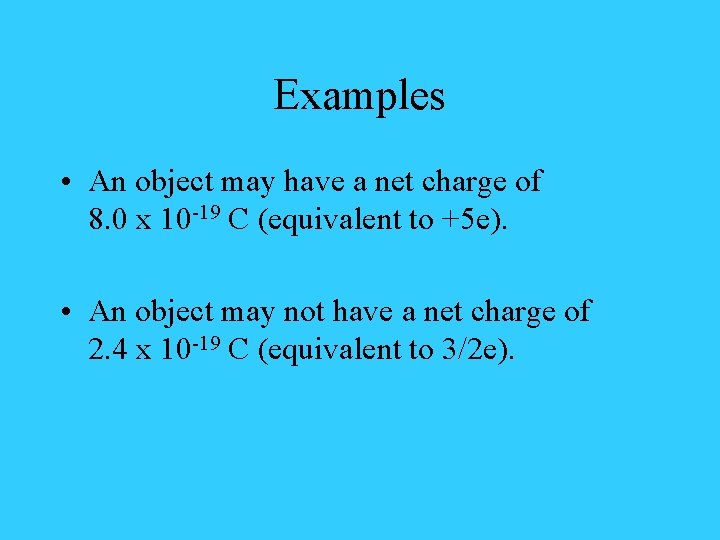
Examples • An object may have a net charge of 8. 0 x 10 -19 C (equivalent to +5 e). • An object may not have a net charge of 2. 4 x 10 -19 C (equivalent to 3/2 e).

Practice • Can an object have a net charge of 9. 6 x 10 -19 C? • Can an object have a net charge of 5. 6 x 10 -19 C? -

Coulomb’s Law • From the comb and paper demo, we can see that the smaller the distance between the two object, the greater the attractive force. • Coulomb determined that the force varies inversely with the square of the distance. • Coulomb also determined that the force varied directly with the charge of the bodies.

Coulomb’s Law • According to Coulombs Law, the electrostatic force, Fe, between two point charges is given by this equation: Fe = kq 1 q 2 / r 2 Where q 1 and q 2 are the charges in coulombs, and r is the separation in meters. The electrostatic constant, k, is equal to 8. 99 x 109 N • m 2 / C 2 (reference tables)

Coulomb’s Law • The equation gives the magnitude of the force that charge q 1 exerts on q 2 and also the force that q 2 exerts on q 1. The two forces are equal in magnitude but opposite in direction. • Electrical force is a vector quantity, like all other force vectors – it needs direction. • A negative sign will indicate a force of attraction, while a positive sign will indicate a repulsive force.
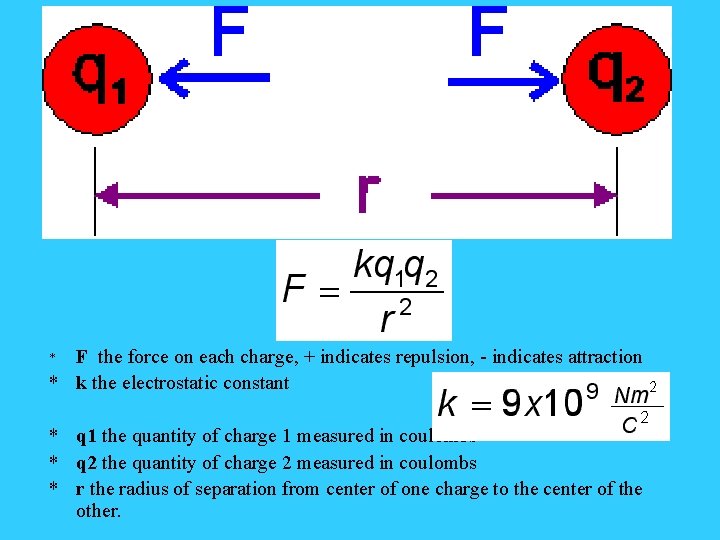
F the force on each charge, + indicates repulsion, - indicates attraction * k the electrostatic constant * * q 1 the quantity of charge 1 measured in coulombs * q 2 the quantity of charge 2 measured in coulombs * r the radius of separation from center of one charge to the center of the other.
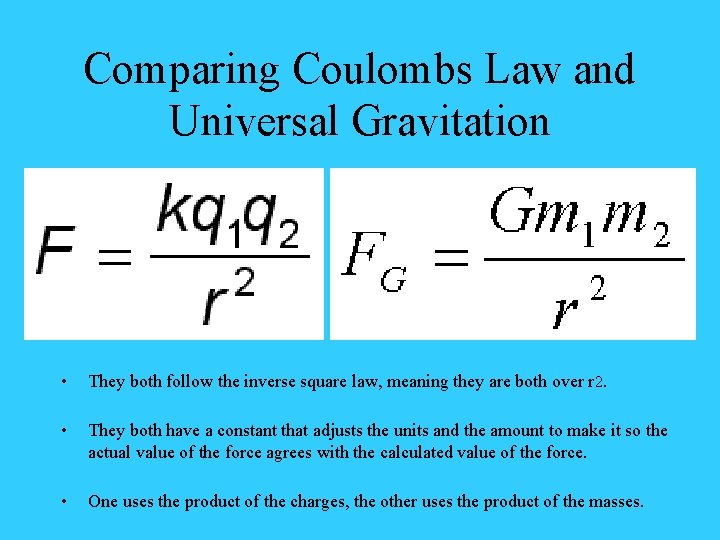
Comparing Coulombs Law and Universal Gravitation • They both follow the inverse square law, meaning they are both over r 2. • They both have a constant that adjusts the units and the amount to make it so the actual value of the force agrees with the calculated value of the force. • One uses the product of the charges, the other uses the product of the masses.

Let’s Practice • Review Book Page 106, #17 • Next, Do #16 • Next Do #12 • Next Do #14
- Slides: 44